Brand Name
Micardis
Generic Name
Telmisartan
View Brand Information FDA approval date: December 01, 2000
Classification: Calcium Channel Blocker
Form: Tablet
What is Micardis (Telmisartan)?
Telmisartan and hydrochlorothiazide tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the classes to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with telmisartan and hydrochlorothiazide tablets, USP. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension , and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects in black patients, and many antihypertensive drugs have additional approved indications and effects . These considerations may guide selection of therapy. Telmisartan and hydrochlorothiazide tablets, USP are not indicated for initial therapy for the treatment of hypertension [see Dosage and Administration.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Micardis HCT (telmisartan and hydrochlorothiazide)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue MICARDIS HCT as soon as possible
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus
1INDICATIONS AND USAGE
MICARDIS HCT (telmisartan and hydrochlorothiazide) is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the classes to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with MICARDIS HCT.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy
MICARDIS HCT is not indicated for initial therapy for the treatment of hypertension
MICARDIS HCT may be used alone or in combination with other antihypertensive agents.
2DOSAGE FORMS AND STRENGTHS
- 40 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H4"
- 80 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H8"
- 80 mg/25 mg, yellow and white tablets marked with the Boehringer Ingelheim logo and "H9"
3CONTRAINDICATIONS
MICARDIS HCT is contraindicated:
- In patients who are hypersensitive to any component of this product
- In patients with anuria.
- For co-administration with aliskiren in patients with diabetes
4ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling:
- Hypotension
- Renal Impairment
- Electrolytes and Metabolic Disorders
4.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
MICARDIS HCT has been evaluated for safety in more than 1700 patients, including 716 treated for hypertension for longer than 6 months and 420 for more than 1 year. Adverse reactions have been limited to those that have been previously reported with telmisartan and/or hydrochlorothiazide.
Adverse reactions occurring at an incidence of ≥2% in patients treated with telmisartan/hydrochlorothiazide and at a greater rate than in patients treated with placebo, are presented in Table 1
Other adverse reactions observed for telmisartan/hydrochlorothiazide were: pain (including back and abdominal), dyspepsia, erythema, vomiting, bronchitis, and pharyngitis.
Adverse reactions occurred at approximately the same rates in men and women, older and younger patients, and black and non-black patients.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of MICARDIS HCT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Eosinophilia
Cardiac Disorders: Tachycardia
Ear and Labyrinth Disorders: Vertigo
General Disorders and Administration Site Conditions: Asthenia, edema
Hepato-biliary: Abnormal hepatic function/liver disorder
Immune System Disorders: Anaphylactic reaction
Investigations: Increased CPK
Metabolism and Nutrition Disorders: Hypoglycemia (in diabetic patients), hyponatremia
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis
Nervous System Disorders: Headache, syncope
Renal and Urinary Disorders: Renal failure, renal impairment including acute renal failure
Reproductive System and Breast Disorders: Erectile dysfunction
Respiratory, Thoracic and Mediastinal Disorders: Coughing
Skin and Subcutaneous Tissue Disorders: Angioedema (with fatal outcome), drug eruption (toxic skin eruption mostly reported as toxicoderma, rash, and urticaria)
Vascular Disorder: Orthostatic hypotension
5DESCRIPTION
MICARDIS HCT tablets are a combination of telmisartan, an orally active angiotensin II antagonist acting on the AT
Telmisartan, a non-peptide molecule, is chemically described as 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empirical formula is C
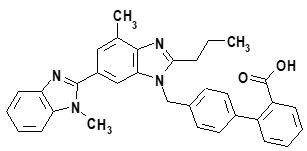
Telmisartan is a white to slightly yellowish solid. It is practically insoluble in water and in the pH range of 3 to 9, sparingly soluble in strong acid (except insoluble in hydrochloric acid), and soluble in strong base.
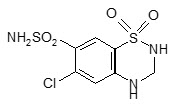
Hydrochlorothiazide is a white, or practically white, practically odorless, crystalline powder with a molecular weight of 297.74. It is slightly soluble in water, and freely soluble in sodium hydroxide solution. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2
MICARDIS HCT tablets are formulated for oral administration in three combinations of 40 mg/12.5 mg, 80 mg/12.5 mg, and 80 mg/25 mg telmisartan and hydrochlorothiazide, respectively. The tablets contain the following inactive ingredients: sodium hydroxide, meglumine, povidone, sorbitol, magnesium stearate, lactose monohydrate, microcrystalline cellulose, maize starch, and sodium starch glycolate. As coloring agents, the 40 mg/12.5 mg and 80 mg/12.5 mg tablets contain ferric oxide red, and the 80 mg/25 mg tablets contain ferric oxide yellow. MICARDIS HCT tablets are hygroscopic and require protection from moisture.
6HOW SUPPLIED/STORAGE AND HANDLING
MICARDIS HCT is available in three strengths as biconvex two-layered, oblong-shaped, uncoated tablets containing telmisartan and hydrochlorothiazide:
- 40 mg/12.5 mg tablet: red and white (may contain red specks) marked with the Boehringer Ingelheim company symbol and "H4"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0043-37)
- 80 mg/12.5 mg tablet: red and white (may contain red specks) marked with the Boehringer Ingelheim company symbol and "H8"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0044-37)
- 80 mg/25 mg tablet: yellow and white (may contain yellow specks) marked with the Boehringer Ingelheim company symbol and "H9"; individually blister-sealed in cartons of 30 tablets as 3 × 10 cards (NDC 0597-0042-37)
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
8Patient Information MICARDIS® HCT (my-CAR-dis HCT) (telmisartan and hydrochlorothiazide tablets)
Read this Patient Information before you start taking MICARDIS HCT tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about MICARDIS HCT tablets?
MICARDIS HCT can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking MICARDIS HCT, tell your doctor right away.
What is MICARDIS HCT?
MICARDIS HCT is a prescription medicine used to treat high blood pressure (hypertension). MICARDIS HCT contains:
- telmisartan, an angiotensin receptor blocker (ARB)
- hydrochlorothiazide, a water pill or diuretic
Your doctor may prescribe other medicines for you to take along with MICARDIS HCT to treat your high blood pressure.
It is not known if MICARDIS HCT is safe and effective in children.
Do not take MICARDIS HCT tablets if you:
- have low or no urine output
- are allergic (hypersensitive) to the active ingredients (telmisartan or hydrochlorothiazide) or any of the other ingredients listed at the end of this leaflet
What should I tell my doctor before using MICARDIS HCT tablets?
Before you take MICARDIS HCT tablets, tell your doctor if you:
- are pregnant or are planning to become pregnant. See
- are breast-feeding or plan to breast-feed. MICARDIS HCT can pass into your breast milk and may harm your baby. You and your doctor should decide if you will take MICARDIS HCT or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take MICARDIS HCT tablets.
- have been told that you have abnormal body salt (electrolytes) levels in your blood
- have liver problems
- have asthma or history of asthma
- have lupus
- have diabetes
- have kidney problems
- have any other medical conditions
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Also, tell your doctor if you drink alcohol.
MICARDIS HCT may affect the way other medicines work, and other medicines may affect how MICARDIS HCT works. Especially tell your doctor if you take:
- aliskiren
- digoxin (Lanoxin
- lithium (Lithobid
- other medicines used to treat your high blood pressure or a heart problem
- water pills (diuretic)
- aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
- potassium supplements or a salt substitute containing potassium
- medicine used to treat diabetes, including insulin
- narcotic pain medicines
- sleeping pills
- steroid medicine or Adrenocorticotrophic Hormone (ACTH)
- barbiturates
- certain cholesterol lowering medicines (resins that are used for cholesterol reduction, e.g., cholestyramine and colestipol resins)
Ask your doctor if you are not sure if you are taking one of the medicines listed above.
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I take MICARDIS HCT tablets?
- Take MICARDIS HCT tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much MICARDIS HCT to take and when to take it.
- Do not change your dose unless your doctor tells you to.
- Take MICARDIS HCT once each day.
- Take MICARDIS HCT tablets with or without food.
- If you take too much MICARDIS HCT, call your doctor, or go to the nearest hospital emergency room right away.
- Read the
What are the possible side effects of MICARDIS HCT tablets?
MICARDIS HCT tablets may cause serious side effects, including:
- Injury or death to your unborn baby. See "
- Low blood pressure (hypotension) is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- do not drink enough fluids
- sweat a lot
- Kidney problems, which may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of MICARDIS HCT tablets. Call your doctor if you get:
- swelling in your feet, ankles, or hands
- unexplained weight gain
- Liver problems, which may get worse in people who already have liver problems and take MICARDIS HCT.
- Eye problems. One of the medicines in MICARDIS HCT can cause eye problems that may lead to vision loss. Symptoms of eye problems can happen within hours to weeks of starting MICARDIS HCT. Tell your doctor right away if you have:
- decrease in vision
- eye pain
- Allergic reactions. Tell your doctor right away if you get any of these symptoms:
- swelling of the face, tongue, throat
- difficulty breathing
- Worsening of lupus. Tell your doctor if your lupus gets worse or becomes active while taking MICARDIS HCT.
- Change in body salts (electrolytes) level in your blood and fluid problems. Your doctor may do tests to check your blood. Call your doctor right away if you have:
- Skin Cancer. One of the medicines in MICARDIS HCT may increase your risk of getting non-melanoma skin cancer. Protect your skin from the sun and undergo regular skin cancer screening when taking MICARDIS HCT.
The most common side effects of MICARDIS HCT tablets include:
- upper respiratory tract infections, including sinus pain/congestion and sore throat
- dizziness
- feeling tired
- flu-like symptoms
- back pain
- diarrhea
- nausea
These are not all the possible side effects with MICARDIS HCT tablets. Tell your doctor if you have any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store MICARDIS HCT tablets?
- Store MICARDIS HCT tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove MICARDIS HCT tablets from blisters until right before you take them.
Keep MICARDIS HCT tablets and all medicines out of the reach of children.
General information about MICARDIS HCT tablets:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MICARDIS HCT tablets for a condition for which it was not prescribed. Do not give MICARDIS HCT tablets to other people, even if they have the same condition you have. It may harm them.
This Patient Information leaflet summarizes the most important information about MICARDIS HCT tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about MICARDIS HCT tablets that is written for health professionals.
For current prescribing information, scan the code or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

What are the ingredients in MICARDIS HCT tablets?
Active Ingredients: telmisartan and hydrochlorothiazide
Inactive Ingredients: sodium hydroxide, meglumine, povidone, sorbitol, magnesium stearate, lactose monohydrate, microcrystalline cellulose, maize starch, and sodium starch glycolate
The 40 mg/12.5 mg and 80 mg/12.5 mg tablets also contain: ferric oxide red.
The 80 mg/25 mg tablets also contain: ferric oxide yellow.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. MICARDIS HCT tablets can help your blood vessels relax so your blood pressure is lower.
How to open the blister:
- Tear (You may also use scissors to tear the blister apart)
- Peel (Peel off the paper layer from the aluminum foil)
- Push (Push the tablet through the aluminum foil)
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Licensed from:
Copyright © 2022 Boehringer Ingelheim International GmbH
MICARDIS
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the ONTARGET
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
Revised: December 2022
COL10674AL272022
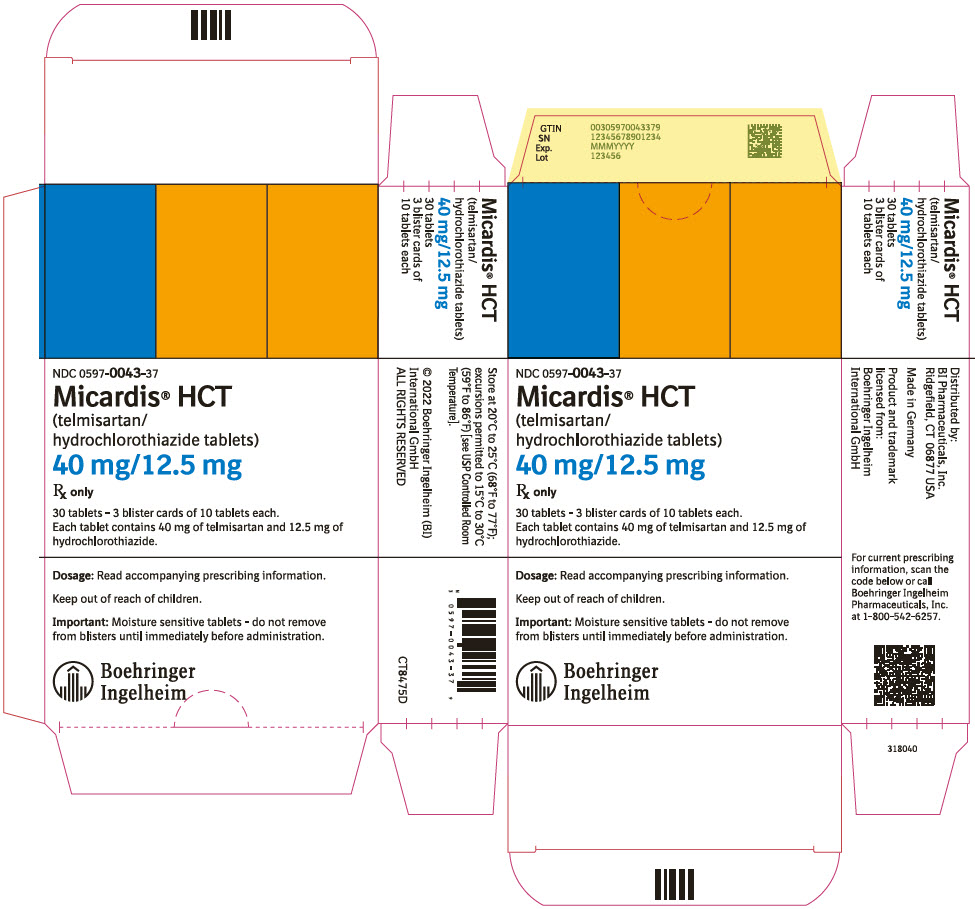
9PRINCIPAL DISPLAY PANEL - 40 mg/12.5 mg Tablet Blister Card Carton
NDC 0597-0043-37
Micardis
40 mg/12.5 mg
Rx only
30 tablets - 3 blister cards of 10 tablets each.
Dosage: Read accompanying prescribing information.
Keep out of reach of children.
Important: Moisture sensitive tablets - do not remove
Boehringer
10PRINCIPAL DISPLAY PANEL - 80 mg/12.5 mg Tablet Blister Card Carton
NDC 0597-0044-37
Micardis
80 mg/12.5 mg
Rx only
30 tablets - 3 blister cards of 10 tablets each.
Dosage: Read accompanying prescribing information.
Keep out of reach of children.
Important: Moisture sensitive tablets - do not remove
Boehringer

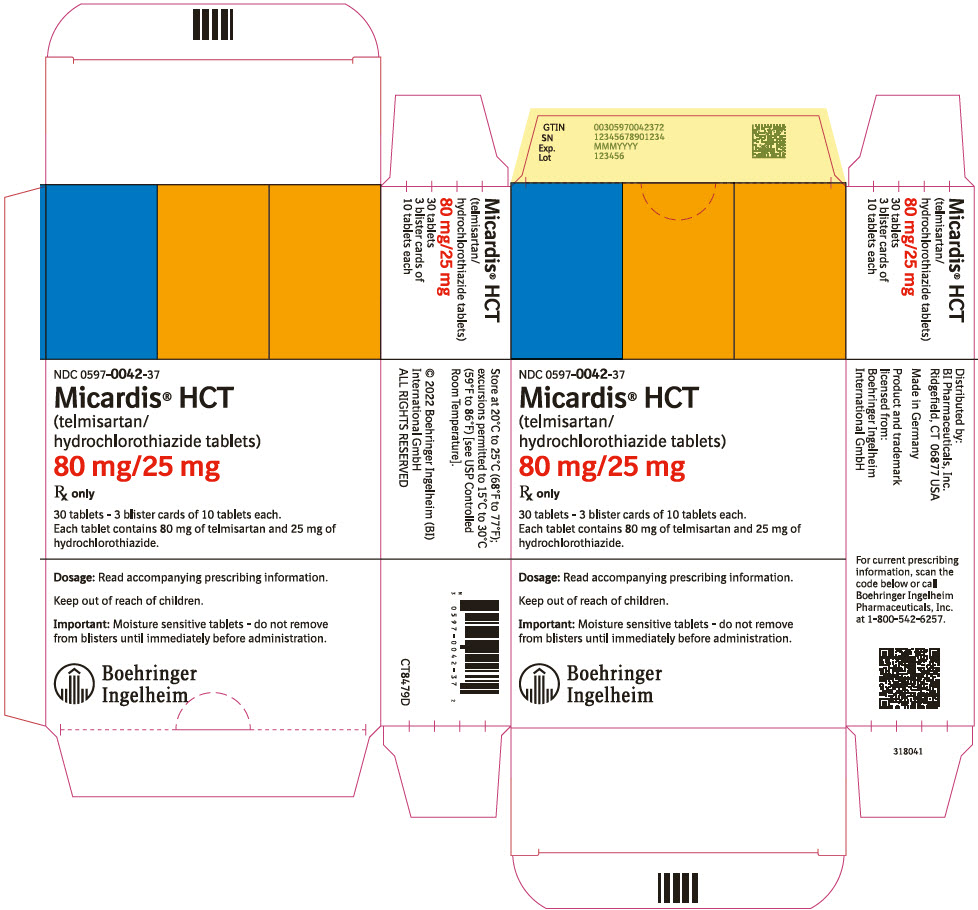
11PRINCIPAL DISPLAY PANEL - 80 mg/25 mg Tablet Blister Card Carton
NDC 0597-0042-37
Micardis
80 mg/25 mg
Rx only
30 tablets - 3 blister cards of 10 tablets each.
Dosage: Read accompanying prescribing information.
Keep out of reach of children.
Important: Moisture sensitive tablets - do not remove
Boehringer