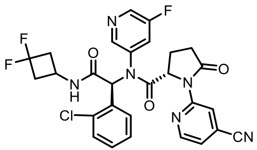
Tibsovo
What is Tibsovo (Ivosidenib)?
Approved To Treat
Related Clinical Trials
Summary: This screening and multi-sub-study Phase 1b/2 trial will establish a method for genomic screening followed by assigning and accruing simultaneously to a multi-study Master Protocol (BAML-16-001-M1). The specific subtype of acute myeloid leukemia will determine which sub-study, within this protocol, a participant will be assigned to evaluate investigational therapies or combinations with the ultima...
Summary: This is a multicenter, non-randomized, umbrella, open-label phase II clinical study, aiming to observe and evaluate, as well as explore the efficacy and safety of precision targeted therapy based on NGS technology for IDH1-mutated patients, specifically the combination of ivosidenib with multi-target tyrosine kinase inhibitors represented by lenvatinib or PD-1/PD-L1 in advanced biliary tract cance...
Summary: This study will enroll participants with myelodysplastic syndromes (MDS) with an Isocitrate dehydrogenase protein, 1 (IDH1) mutation, who have not received treatment with a hypomethylating agent previously. Participants will be randomized to receive either ivosidenib (IVO) alone or azacitidine (AZA) alone. IVO will be administered daily throughout the 28-day treatment cycle and AZA will be adminis...
Related Latest Advances
Brand Information
- Differentiation Syndrome in AML and MDS
- QTc Interval Prolongation
- Guillain-Barré Syndrome