Generic Name
PegFilgrastim
Brand Names
Nyvepria, Udenyca, Fulphila, Neulasta, Ziextenzo, Stimufend, Fylnetra
FDA approval date: April 01, 2002
Classification: Leukocyte Growth Factor
Form: Injection, Kit
What is Nyvepria (PegFilgrastim)?
Stimufend is a leukocyte growth factor indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
NYVEPRIA (pegfilgrastim-apgf)
1INDICATIONS AND USAGE
NYVEPRIA is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia
2DOSAGE FORMS AND STRENGTHS
NYVEPRIA is a clear, colorless, preservative-free solution available as:
- Injection: 6 mg/0.6 mL in a single-dose prefilled syringe for manual use only.
3CONTRAINDICATIONS
NYVEPRIA is contraindicated in patients with a history of serious allergic reactions to pegfilgrastim products or filgrastim products. Reactions have included anaphylaxis
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Splenic Rupture
- Acute Respiratory Distress Syndrome
- Serious Allergic Reactions
- Use in Patients with Sickle Cell Disorders
- Glomerulonephritis
- Leukocytosis
- Thrombocytopenia
- Capillary Leak Syndrome
- Potential for Tumor Growth Stimulatory Effects on Malignant Cells
- Myelodysplastic Syndrome
- Acute Myeloid Leukemia
- Aortitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pegfilgrastim clinical trials safety data are based upon 932 patients receiving pegfilgrastim in seven randomized clinical trials. The population was 21 to 88 years of age and 92% female. The ethnicity was 75% Caucasian, 18% Hispanic, 5% Black, and 1% Asian. Patients with breast (n = 823), lung and thoracic tumors (n = 53) and lymphoma (n = 56) received pegfilgrastim after nonmyeloablative cytotoxic chemotherapy. Most patients received a single 100 mcg/kg (n = 259) or a single 6 mg (n = 546) dose per chemotherapy cycle over 4 cycles.
The following adverse reaction data in Table 2 are from a randomized, double-blind, placebo-controlled study in patients with metastatic or non-metastatic breast cancer receiving docetaxel 100 mg/m
The most common adverse reactions occurring in ≥5% of patients and with a between-group difference of ≥5% higher in the pegfilgrastim arm in placebo-controlled clinical trials are bone pain and pain in extremity.
4.2Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other pegfilgrastim products may be misleading.
Binding antibodies to pegfilgrastim were detected using a BIAcore assay. The approximate limit of detection for this assay is 500 ng/mL. Pre-existing binding antibodies were detected in approximately 6% (51/849) of patients with metastatic breast cancer. Four of 521 pegfilgrastim-treated subjects who were negative at baseline developed binding antibodies to pegfilgrastim following treatment. None of these 4 patients had evidence of neutralizing antibodies detected using a cell-based bioassay.
4.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pegfilgrastim products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Splenic rupture and splenomegaly (enlarged spleen)
- Acute respiratory distress syndrome (ARDS)
- Allergic reactions/hypersensitivity, including anaphylaxis, skin rash, urticaria, generalized erythema, and flushing
- Sickle cell crisis
- Glomerulonephritis
- Leukocytosis
- Thrombocytopenia
- Capillary Leak Syndrome
- Injection site reactions
- Sweet's syndrome (acute febrile neutrophilic dermatosis), cutaneous vasculitis
- Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with breast and lung cancer receiving chemotherapy and/or radiotherapy
- Aortitis
- Alveolar hemorrhage
5OVERDOSAGE
Overdosage of pegfilgrastim products may result in leukocytosis and bone pain. Events of edema, dyspnea, and pleural effusion have been reported in a single patient who administered pegfilgrastim on 8 consecutive days in error. In the event of overdose, the patient should be monitored for adverse reactions
6DESCRIPTION
Pegfilgrastim-apgf is a covalent conjugate of recombinant methionyl human G-CSF and monomethoxypolyethylene glycol. Recombinant methionyl human G-CSF is a water-soluble 175 amino acid protein with a molecular weight of approximately 19 kilodaltons (kD). Recombinant methionyl human G-CSF is obtained from the bacterial fermentation of a strain of
NYVEPRIA for manual subcutaneous injection is supplied in 0.6 mL prefilled syringes. The prefilled syringe does not bear graduation marks and is designed to deliver the entire contents of the syringe (6 mg/0.6 mL).
The delivered 0.6 mL dose from the prefilled syringe for manual subcutaneous injection contains 6 mg pegfilgrastim-apgf (based on protein weight) in a sterile, clear, colorless, preservative-free solution (pH 4.0) containing acetate (0.35 mg), polysorbate 20 (0.02 mg), sodium (0.01 mg), and sorbitol (30 mg) in Water for Injection, USP.
7CLINICAL STUDIES
Pegfilgrastim was evaluated in three randomized, double-blind, controlled studies. Studies 1 and 2 were active-controlled studies that employed doxorubicin 60 mg/m
In Study 1, 157 patients were randomized to receive a single subcutaneous injection of pegfilgrastim (6 mg) on day 2 of each chemotherapy cycle or daily subcutaneous filgrastim (5 mcg/kg/day) beginning on day 2 of each chemotherapy cycle. In Study 2, 310 patients were randomized to receive a single subcutaneous injection of pegfilgrastim (100 mcg/kg) on day 2 or daily subcutaneous filgrastim (5 mcg/kg/day) beginning on day 2 of each chemotherapy cycle.
Both studies met the major efficacy outcome measure of demonstrating that the mean days of severe neutropenia of pegfilgrastim-treated patients did not exceed that of filgrastim-treated patients by more than 1 day in cycle 1 of chemotherapy. The mean days of cycle 1 severe neutropenia in Study 1 were 1.8 days in the pegfilgrastim arm compared to 1.6 days in the filgrastim arm [difference in means 0.2 (95% CI -0.2, 0.6)] and in Study 2 were 1.7 days in the pegfilgrastim arm compared to 1.6 days in the filgrastim arm [difference in means 0.1 (95% CI -0.2, 0.4)].
A secondary endpoint in both studies was days of severe neutropenia in cycles 2 through 4 with results similar to those for cycle 1.
Study 3 was a randomized, double-blind, placebo-controlled study that employed docetaxel 100 mg/m
Study 4 was a multicenter, randomized, open-label study to evaluate the efficacy, safety, and pharmacokinetics
8HOW SUPPLIED/STORAGE AND HANDLING
NYVEPRIA (pegfilgrastim-apgf) injection is a clear, colorless solution supplied in a prefilled single-dose syringe for manual use containing 6 mg pegfilgrastim-apgf, supplied with a 27-gauge 1/2-inch needle and a BD UltraSafe Plus™ Passive Needle Guard.
The NYVEPRIA syringe plunger stopper and needle cover are not made with natural rubber latex.
NYVEPRIA is provided in a dispensing pack containing one sterile 6 mg/0.6 mL prefilled syringe (NDC 0069-0324-01).
NYVEPRIA prefilled syringe does not bear graduation marks and is intended only to deliver the entire contents of the syringe (6 mg/0.6 mL) for direct administration. Use of the prefilled syringe is not recommended for direct administration for pediatric patients weighing less than 45 kg who require doses that are less than the full contents of the syringe.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Advise patients of the following risks and potential risks with NYVEPRIA:
- Splenic rupture and splenomegaly
- Acute Respiratory Distress Syndrome
- Serious allergic reactions
- Sickle cell crisis
- Glomerulonephritis
- Increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with breast and lung cancer who receive pegfilgrastim products in conjunction with chemotherapy and/or radiation therapy
- Capillary Leak Syndrome
- Aortitis
Instruct patients who self-administer NYVEPRIA using the single-dose prefilled syringe of the:
- Importance of following the Instructions for Use.
- Dangers of reusing syringes.
- Importance of following local requirements for proper disposal of used syringes.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
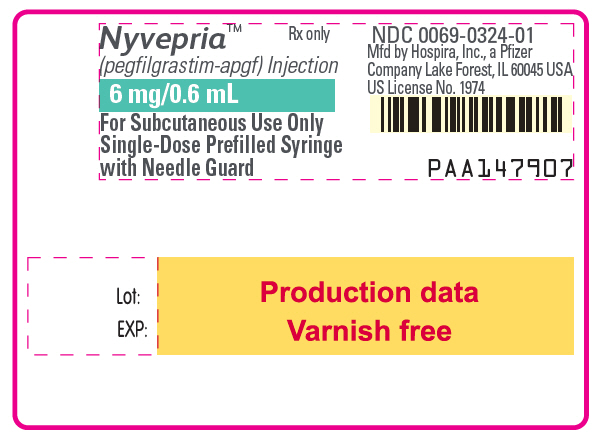
10PRINCIPAL DISPLAY PANEL - 6 mg/0.6 mL Syringe Label
Nyvepria™
Rx only
6 mg/0.6 mL
For Subcutaneous Use Only
NDC 0069-0324-01
PAA147907
11PRINCIPAL DISPLAY PANEL - 6 mg/0.6 mL Syringe Inner Carton – PAA207017
Nyvepria™
6 mg/0.6 mL
For Subcutaneous Use Only
1 x 6 mg/0.6 mL
Pegylated Recombinant Methionyl Human
NDC 0069-0324-01
Rx Only

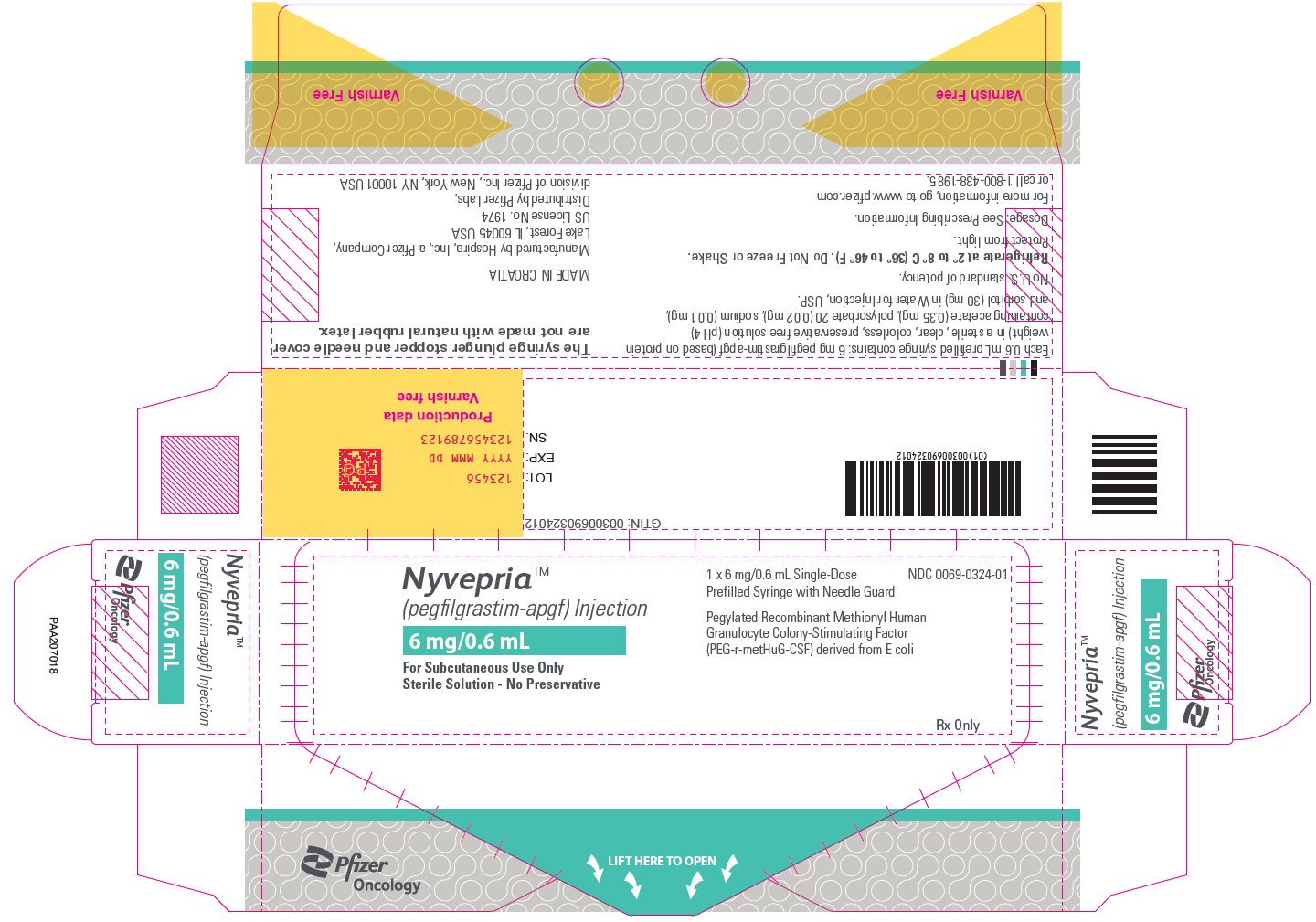
12PRINCIPAL DISPLAY PANEL - 6 mg/0.6 mL Syringe Outer Carton – PAA207018
Nyvepria™
6 mg/0.6 mL
For Subcutaneous Use Only
1 x 6 mg/0.6 mL Single-Dose
Pegylated Recombinant Methionyl Human
NDC 0069-0324-01
Rx Only