Brand Name
Zavzpret
Generic Name
Zavegepant
View Brand Information FDA approval date: June 01, 2023
Classification: Calcitonin Gene-related Peptide Receptor Antagonist
Form: Spray
What is Zavzpret (Zavegepant)?
ZAVZPRET is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use ZAVZPRET is not indicated for the preventive treatment of migraine. ZAVZPRET is a calcitonin gene-related peptide receptor antagonist indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use ZAVZPRET is not indicated for the preventive treatment of migraine.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ZAVZPRET (zavegepant)
1INDICATIONS AND USAGE
ZAVZPRET is indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use
ZAVZPRET is not indicated for the preventive treatment of migraine.
2DOSAGE FORMS AND STRENGTHS
Nasal spray: 10 mg of zavegepant per device. Each unit-dose nasal spray device delivers a single spray containing 10 mg of zavegepant.
3CONTRAINDICATIONS
ZAVZPRET is contraindicated in patients with a history of hypersensitivity reaction to zavegepant or any of the components of ZAVZPRET.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypersensitivity Reactions
- Hypertension
- Raynaud’s Phenomenon
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ZAVZPRET for the acute treatment of migraine in adults has been evaluated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in patients with migraine who received one 10 mg dose of ZAVZPRET nasal spray (N=1023) or placebo (N=1056)
Adverse reactions in Study 1 and 2 are shown in Table 1.
Table 1: Adverse Reactions Occurring in At Least 2% of Patients Treated with ZAVZPRET and at a Frequency Greater than Placebo in Study 1 and 2
Hypersensitivity, including facial swelling and urticaria, occurred in less than 1% of patients treated with ZAVZPRET
Long-term safety was assessed in an open-label extension study. That study evaluated 603 patients, dosing intermittently for up to one year, including 360 patients who were exposed to ZAVZPRET 10 mg for at least 6 months, and 298 who were exposed for at least one year, all of whom treated an average of at least two migraine attacks per month.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ZAVZPRET. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity (e.g., anaphylaxis) [see
Vascular Disorders: Hypertension [see , Raynaud’s phenomenon [see
5DESCRIPTION
ZAVZPRET (zavegepant) nasal spray contains zavegepant hydrochloride, a calcitonin gene-related peptide receptor antagonist. Zavegepant hydrochloride is described chemically as (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl) piperazin-1-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl) piperidine-1-carboxamide hydrochloride and its structural formula is:
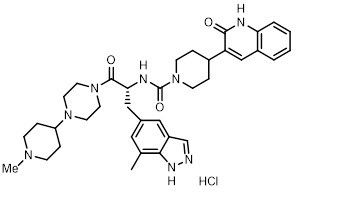
Its molecular formula is C
Each unit-dose ZAVZPRET device for nasal administration delivers 10 mg of zavegepant (equivalent to 10.6 mg of zavegepant hydrochloride) in a buffered aqueous solution containing dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection. The solution has a pH of 5.3 to 6.7.
6CLINICAL STUDIES
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2: Efficacy Endpoints in Study 1
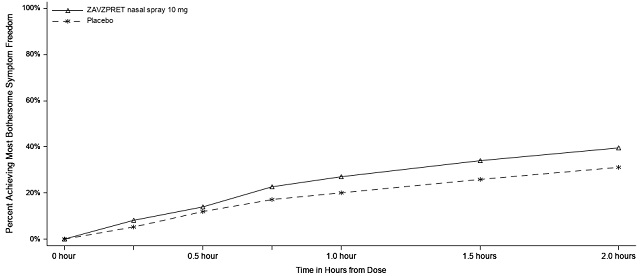
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1
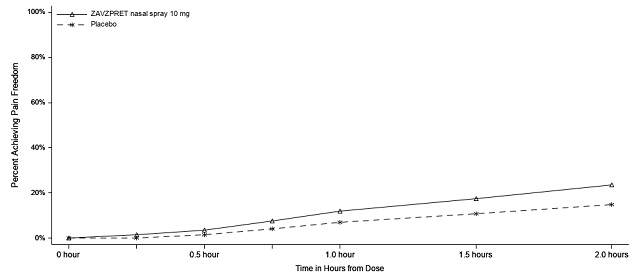
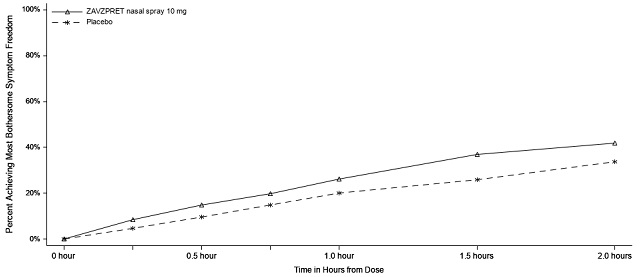
Figure 2: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3: Additional Efficacy Endpoints in Study 1
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value = 0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value = 0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4: Efficacy Endpoints in Study 2
Figure 3: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2
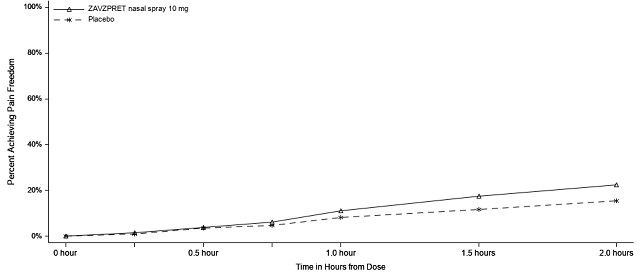
Figure 4: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2
7PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions, and that these reactions can occur after administration of ZAVZPRET. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur
Hypertension
Inform patients that hypertension can develop or pre-existing hypertension can worsen with ZAVZPRET, and that they should contact their healthcare provider if they experience elevation in their blood pressure
Raynaud’s Phenomenon
Inform patients that Raynaud’s phenomenon can develop or worsen with ZAVZPRET. Advise patients to discontinue ZAVZPRET and contact their healthcare provider if they experience signs or symptoms of Raynaud’s phenomenon
Drug Interactions
Advise patients to speak with their healthcare provider about any prescription or over-the-counter medications or herbal supplements that they take or plan to take. Inform patients that if they need to use an intranasal decongestant it should be administered at least 1 hour after ZAVZPRET administration
This product’s labeling may have been updated. For the most recent prescribing information, please visit

LAB-1544-3.0
8Patient Package Insert
What is ZAVZPRET?
ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks with or without aura.
ZAVZPRET is not used to prevent migraine attacks.
It is not known if ZAVZPRET is safe and effective in children.
Do not use ZAVZPRET if you are:
- allergic to zavegepant, or any of the ingredients in ZAVZPRET.
See the end of this leaflet for a complete list of ingredients in ZAVZPRET.
Before you use ZAVZPRET, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure.
- have circulation problems in your fingers and toes.
- have kidney problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if ZAVZPRET will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known whether ZAVZPRET passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you use ZAVZPRET.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ZAVZPRET?
- Use ZAVZPRET exactly how your healthcare provider tells you to use it.
- See the Instructions for Use for complete information on how to use ZAVZPRET nasal spray.
- ZAVZPRET is given in the nose (nasal) only.
- Each ZAVZPRET only sprays 1 time and cannot be reused.
- Each dose of ZAVZPRET is provided in an individual pack. Use all of the medicine in 1 pack for a complete dose.
- The recommended dose is 10 mg given as a single spray in one nostril.
- Do not use more than 1 spray (10 mg) of ZAVZPRET nasal spray in a 24-hour period.
- It is not known if it is safe to use more than 8 sprays (doses) of ZAVZPRET in 30 days.
- Avoid using intranasal decongestants with ZAVZPRET. If you have to use an intranasal decongestant, use it at least 1 hour after using ZAVZPRET.
What are the possible side effects of ZAVZPRET?
ZAVZPRET may cause serious side effects including:
- Allergic reactions. Allergic reactions, including trouble breathing, hives, and swelling of the face, can happen after you use ZAVZPRET. Call your healthcare provider or get emergency help right away if you have any of the following symptoms, which may be part of an allergic reaction:
- swelling of the face, mouth, tongue, or throat
- trouble breathing
- rash
- hives
- High blood pressure. High blood pressure or worsening of high blood pressure can happen after you use ZAVZPRET. Contact your healthcare provider if you have an increase in blood pressure.
- Raynaud’s phenomenon. A type of circulation problem can worsen or happen after you use ZAVZPRET. Raynaud’s phenomenon can lead to your fingers or toes feeling numb, cool, or painful, or changing color from pale, to blue, to red. Contact your healthcare provider if these symptoms occur.
The most common side effects of ZAVZPRET are:
- unusual taste
- nausea
- nasal discomfort
- vomiting
These are not the only possible side effects of ZAVZPRET.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZAVZPRET?
- Store ZAVZPRET in the blister package that it comes in.
- Store ZAVZPRET at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze.
Keep ZAVZPRET and all medicines out of the reach of children.
General information about the safe and effective use of ZAVZPRET:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZAVZPRET for a condition for which it was not prescribed. Do not give ZAVZPRET to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZAVZPRET that is written for health professionals.
What are the ingredients in ZAVZPRET?
Active ingredients in ZAVZPRET: zavegepant
Inactive ingredients in ZAVZPRET: dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection.

For more information, go to
LAB-1545-3.0
9PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Front Sample
Zavzpret
PROFESSIONAL SAMPLE –NOT FOR SALE
Do not test spray,

10PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Back Sample
U.S. Pharmaceuticals
LOT xxxxxxx
11PRINCIPAL DISPLAY PANEL – Nasal Spray Blister Foil Label Sample
NDC 63539-135-01
PROFESSIONAL SAMPLE –
PEEL OFF
Zavzpret
Each unit-dose device contains 10 mg of zavegepant.
Pfizer

12PRINCIPAL DISPLAY PANEL – Nasal Spray Carton Sample
PROFESSIONAL
NDC 63539-135-02
Zavzpret
This box contains:
For Intranasal use only.
1 spray (10 mg dose) per unit.
