Generic Name
Dantrolene
Brand Names
Dantrium, Revonto, RYANODEX Dantrolene
FDA approval date: March 01, 2005
Classification: Skeletal Muscle Relaxant
Form: Injection, Capsule
What is Dantrium (Dantrolene)?
In Chronic Spasticity: Dantrolene sodium is indicated in controlling the manifestations of clinical spasticity resulting from upper motor neuron disorders . It is of particular benefit to the patient whose functional rehabilitation has been retarded by the sequelae of spasticity. Such patients must have presumably reversible spasticity where relief of spasticity will aid in restoring residual function. Dantrolene sodium is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders. If improvement occurs, it will ordinarily occur within the dosage titration, and will be manifested by a decrease in the severity of spasticity and the ability to resume a daily function not quite attainable without dantrolene sodium. Occasionally, subtle but meaningful improvement in spasticity may occur with dantrolene sodium therapy. In such instances, information regarding improvement should be solicited from the patient and those who are in constant daily contact and attendance with him. Brief withdrawal of dantrolene sodium for a period of 2 to 4 days will frequently demonstrate exacerbation of the manifestations of spasticity and may serve to confirm a clinical impression. A decision to continue the administration of dantrolene sodium on a long-term basis is justified if introduction of the drug into the patient's regimen: produces a significant reduction in painful and/or disabling spasticity such as clonus, or permits a significant reduction in the intensity and/or degree of nursing care required, or rids the patient of any annoying manifestation of spasticity considered important by the patient himself. In Malignant Hyperthermia: Oral dantrolene sodium is also indicated preoperatively to prevent or attenuate the development of signs of malignant hyperthermia in known, or strongly suspect, malignant hyperthermia susceptible patients who require anesthesia and/or surgery. Currently accepted clinical practices in the management of such patients must still be adhered to ; see also the package insert for Dantrium ® Intravenous. Oral dantrolene sodium should be administered following a malignant hyperthermic crisis to prevent recurrence of the signs of malignant hyperthermia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Dantrium (dantrolene sodium)
1DESCRIPTION:
Dantrium Intravenous is a sterile, non-pyrogenic, lyophilized formulation of dantrolene sodium for injection. Dantrium Intravenous is supplied in 70 mL vials containing 20 mg dantrolene sodium, 3000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
Dantrium is classified as a direct-acting skeletal muscle relaxant. Chemically, Dantrium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2,4-imidazolidinedione sodium salt. The structural formula for the hydrated salt is:
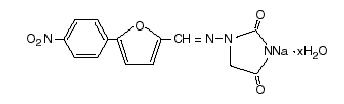
The hydrated salt contains approximately 15% water (3-1/2 moles) and has a molecular weight of 399. The anhydrous salt (dantrolene) has a molecular weight of 336.
2CLINICAL PHARMACOLOGY:
In isolated nerve-muscle preparation,
In the anesthetic-induced malignant hyperthermia syndrome, evidence points to an intrinsic abnormality of skeletal muscle tissue. In affected humans, it has been postulated that "triggering agents" (e.g., general anesthetics and depolarizing neuromuscular blocking agents) produce a change within the cell which results in an elevated myoplasmic calcium. This elevated myoplasmic calcium activates acute cellular catabolic processes that cascade to the malignant hyperthermia crisis.
It is hypothesized that addition of
Specific metabolic pathways for the degradation and elimination of
The mean biologic half-life of
Cardiopulmonary depression has not been observed in malignant hyperthermia susceptible swine following the administration of up to 7.5 mg/kg intravenous dantrolene. This is twice the amount needed to maximally diminish twitch response to single supramaximal peripheral nerve stimulation (95% inhibition). A transient, inconsistent, depressant effect on gastrointestinal smooth muscles has been observed at high doses.
3INDICATIONS AND USAGE:
Dantrium Intravenous is indicated, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of malignant hyperthermia crises in patients of all ages. Dantrium Intravenous should be administered by continuous rapid intravenous push as soon as the malignant hyperthermia reaction is recognized (i.e., tachycardia, tachypnea, central venous desaturation, hypercarbia, metabolic acidosis, skeletal muscle rigidity, increased utilization of anesthesia circuit carbon dioxide absorber, cyanosis and mottling of the skin, and, in many cases, fever).
Dantrium Intravenous is also indicated preoperatively, and sometimes postoperatively, to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia in individuals judged to be malignant hyperthermia susceptible.
4CONTRAINDICATIONS:
None.
5WARNINGS:
The use of
Since the effect of disease state and other drugs on
If patients judged malignant hyperthermia susceptible are administered intravenous or oral
6ADVERSE REACTIONS:
There have been occasional reports of death following malignant hyperthermia crisis even when treated with intravenous dantrolene; incidence figures are not available (the pre-dantrolene mortality of malignant hyperthermia crisis was approximately 50%). Most of these deaths can be accounted for by late recognition, delayed treatment, inadequate dosage, lack of supportive therapy, intercurrent disease and/or the development of delayed complications such as renal failure or disseminated intravascular coagulopathy. In some cases there are insufficient data to completely rule out therapeutic failure of dantrolene.
There are reports of fatality in malignant hyperthermia crisis, despite initial satisfactory response to intravenous dantrolene, which involve patients who could not be weaned from dantrolene after initial treatment.
The administration of
The following adverse reactions are in approximate order of severity:
There are rare reports of pulmonary edema developing during the treatment of malignant hyperthermia crisis in which the diluent volume and mannitol needed to deliver intravenous dantrolene possibly contributed.
There have been reported cases of hepatotoxicity following the use of intravenous dantrolene products. Elevated liver enzymes have occurred hours to days following use of intravenous dantrolene, though many of these cases were observed in patients with comorbidities (e.g., critical illness).
There have been reports of thrombophlebitis following administration of intravenous dantrolene; actual incidence figures are not available. Tissue necrosis secondary to extravasation has been reported.
There have been rare reports of urticaria and erythema possibly associated with the administration of
Injection site reactions (pain, erythema, swelling), commonly due to extravasation, have been reported.
The following events have been reported in patients receiving oral dantrolene: aplastic anemia, leukopenia, lymphocytic lymphoma, and heart failure. (See package insert for
The published literature has included some reports of
For medical advice about adverse reactions contact your medical professional. To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
7OVERDOSAGE:
Because
Symptoms which may occur in case of overdose include, but are not limited to, muscular weakness and alterations in the state of consciousness (e.g., lethargy, coma), vomiting, diarrhea, and crystalluria.
For acute overdosage, general supportive measures should be employed.
Intravenous fluids should be administered in fairly large quantities to avert the possibility of crystalluria. An adequate airway should be maintained and artificial resuscitation equipment should be at hand. Electrocardiographic monitoring should be instituted, and the patient carefully observed. The value of dialysis in
8DOSAGE AND ADMINISTRATION:
As soon as the malignant hyperthermia reaction is recognized, all anesthetic agents should be discontinued; the administration of 100% oxygen is recommended.
If the physiologic and metabolic abnormalities reappear, the regimen may be repeated. It is important to note that administration of
8.1Pediatric Dose:
Experience to date indicates that the dose of
8.2Preoperatively:
Dantrium Intravenous and/or Dantrium Capsules may be administered preoperatively to patients judged malignant hyperthermia susceptible as part of the overall patient management to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia.
8.2.1
Dantrium Intravenous: The recommended prophylactic dose of Dantrium Intravenous is 2.5 mg/kg, starting approximately 1-1/4 hours before anticipated anesthesia and infused over approximately 1 hour. This dose should prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia provided that the usual precautions, such as avoidance of established malignant hyperthermia triggering agents, are followed.
Additional
8.2.2Oral Administration of Dantrium Capsules:
Administer 4 to 8 mg/kg/day of oral
8.3Post Crisis Follow-Up:
Dantrium Capsules, 4 to 8 mg/kg/day, in four divided doses should be administered for 1 to 3 days following a malignant hyperthermia crisis to prevent recurrence of the manifestations of malignant hyperthermia.
Dantrium Intravenous may be used postoperatively to prevent or attenuate the recurrence of signs of malignant hyperthermia when oral administration of Dantrium Capsules is not practical. The dose of Dantrium Intravenous in the postoperative period must be individualized, starting with 1 mg/kg or more as the clinical situation dictates.
8.4PREPARATION:
Each vial of
Reconstituted
For prophylactic infusion, the required number of individual vials of
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
9HOW SUPPLIED:
Dantrium Intravenous (NDC 42023-123-06) is available in vials containing a sterile lyophilized mixture of 20 mg dantrolene sodium, 3000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
10PRINCIPAL DISPLAY PANEL - 20 mg Vial Carton
6 Vials Nonreturnable
20 mg
Dantrium
(dantrolene sodium for injection)
(dantrolene sodium for injection)
For treatment of malignant hyperthermia
FOR INTRAVENOUS USE ONLY
FOR INTRAVENOUS USE ONLY
Rx Only
