Generic Name
Timolol
Brand Names
Betimol, Timoptic-XE, Timoptic, Istalol, Timolol GFS
FDA approval date: November 05, 1986
Classification: beta-Adrenergic Blocker
Form: Tablet, Solution
What is Betimol (Timolol)?
Timolol maleate ophthalmic gel forming solution is indicated for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. Timolol maleate is a beta adrenergic blocker indicated for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
BETIMOL (timolol)
1DESCRIPTION
Betimol
Timolol (as the hemihydrate) is a white, odorless, crystalline powder which is slightly soluble in water and freely soluble in ethanol. Timolol hemihydrate is stable at room temperature.
Betimol
It is supplied in two dosage strengths, 0.25% and 0.5%.
Each mL of Betimol
Each mL of Betimol
Inactive ingredients: monosodium and disodium phosphate dihydrate to adjust pH (6.5 - 7.5) and water for injection, benzalkonium chloride 0.01 % added as preservative.
The osmolality of Betimol
2CLINICAL PHARMACOLOGY
Timolol is a non-selective beta-adrenergic antagonist.
It blocks both beta
Timolol, when applied topically in the eye, reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. Elevated intraocular pressure is a major risk factor in the pathogenesis of glaucomatous visual field loss. The higher the level of IOP, the greater the likelihood of glaucomatous visual field loss and optic nerve damage. The predominant mechanism of ocular hypotensive action of topical beta-adrenergic blocking agents is likely due to a reduction in aqueous humor production.
In general, beta-adrenergic blocking agents reduce cardiac output both in healthy subjects and patients with heart diseases. In patients with severe impairment of myocardial function, beta-adrenergic receptor blocking agents may inhibit sympathetic stimulatory effect necessary to maintain adequate cardiac function. In the bronchi and bronchioles, beta-adrenergic receptor blockade may also increase airway resistance because of unopposed parasympathetic activity.
2.1Pharmacokinetics
When given orally, timolol is well absorbed and undergoes considerable first pass metabolism. Timolol and its metabolites are primarily excreted in the urine. The half-life of timolol in plasma is approximately 4 hours.
2.2Clinical Studies
In two controlled multicenter studies in the U.S., Betimol
3INDICATIONS AND USAGE
Betimol
4CONTRAINDICATIONS
Betimol
5WARNINGS
As with other topically applied ophthalmic drugs, Betimol
5.1Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe cardiac failure.
In patients without a history of cardiac failure, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. Betimol
5.2Obstructive Pulmonary Disease
Patients with chronic obstructive pulmonary disease (e.g. chronic bronchitis, emphysema) of mild or moderate severity, bronchospastic disease, or a history of bronchospastic disease (other than bronchial asthma or a history of bronchial asthma which are contraindications) should in general not receive beta-blocking agents.
5.3Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to a major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic receptor blocking agents is recommended. If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of beta-adrenergic agonists.
5.4Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
5.5Thyrotoxicosis
Beta-adrenergic blocking agents may mask certain clinical signs (e.g. tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blocking agents which might precipitate a thyroid storm.
6ADVERSE REACTIONS
The most frequently reported ocular event in clinical trials was burning/stinging on instillation and was comparable between Betimol
The following adverse events were associated with use of Betimol
OCULAR:
Dry eyes, itching, foreign body sensation, discomfort in the eye, eyelid erythema, conjunctival injection, and headache.
BODY AS A WHOLE:
Headache.
The following side effects were reported in frequencies of 1 to 5%:
OCULAR:
Eye pain, epiphora, photophobia, blurred or abnormal vision, corneal fluorescein staining, keratitis, blepharitis and cataract.
BODY AS A WHOLE:
Allergic reaction, asthenia, common cold and pain in extremities.
CARDIOVASCULAR:
Hypertension.
DIGESTIVE:
Nausea.
METABOLIC/NUTRITIONAL:
Peripheral edema.
NERVOUS SYSTEM/PSYCHIATRY:
Dizziness and dry mouth.
RESPIRATORY:
Respiratory infection and sinusitis.
In addition, the following adverse reactions have been reported with ophthalmic use of beta blockers:
OCULAR:
Conjunctivitis, blepharoptosis, decreased corneal sensitivity, visual disturbances including refractive changes, diplopia and retinal vascular disorder.
BODY AS A WHOLE:
Chest pain.
CARDIOVASCULAR:
Arrhythmia, palpitation, bradycardia, hypotension, syncope, heart block, cerebral vascular accident, cerebral ischemia, cardiac failure and cardiac arrest.
DIGESTIVE:
Diarrhea.
ENDOCRINE:
Masked symptoms of hypoglycemia in insulin dependent diabetics (See
NERVOUS SYSTEM/PSYCHIATRY:
Depression, impotence, increase in signs and symptoms of myasthenia gravis and paresthesia.
RESPIRATORY:
Dyspnea, bronchospasm, respiratory failure and nasal congestion.
SKIN:
Alopecia, hypersensitivity including localized and generalized rash, urticaria.
7OVERDOSAGE
No information is available on overdosage with Betimol
8DOSAGE AND ADMINISTRATION
Betimol® Ophthalmic Solution is available in concentrations of 0.25% and 0.5%. The usual starting dose is one drop of 0.25% Betimol® in the affected eye(s) twice a day. If the clinical response is not adequate, the dosage may be changed to one drop of 0.5% solution in the affected eye(s) twice a day.
If the intraocular pressure is maintained at satisfactory levels, the dosage schedule may be changed to one drop once a day in the affected eye(s). Because of diurnal variations in intraocular pressure, satisfactory response to the once-a-day dose is best determined by measuring the intraocular pressure at different times during the day.
Since in some patients the pressure-lowering response to Betimol® may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with Betimol®.
Dosages above one drop of 0.5% Betimol® twice a day generally have not been shown to produce further reduction in intraocular pressure. If the patient's intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy with pilocarpine and other miotics, and/or epinephrine, and/or systemically administered carbonic anhydrase inhibitors, such as acetazolamide, can be instituted.
9HOW SUPPLIED
Betimol
Betimol
Betimol
Rx Only
9.1STORAGE
Store between 15° to 25°C (59° to 77°F). Do not freeze. Protect from light.
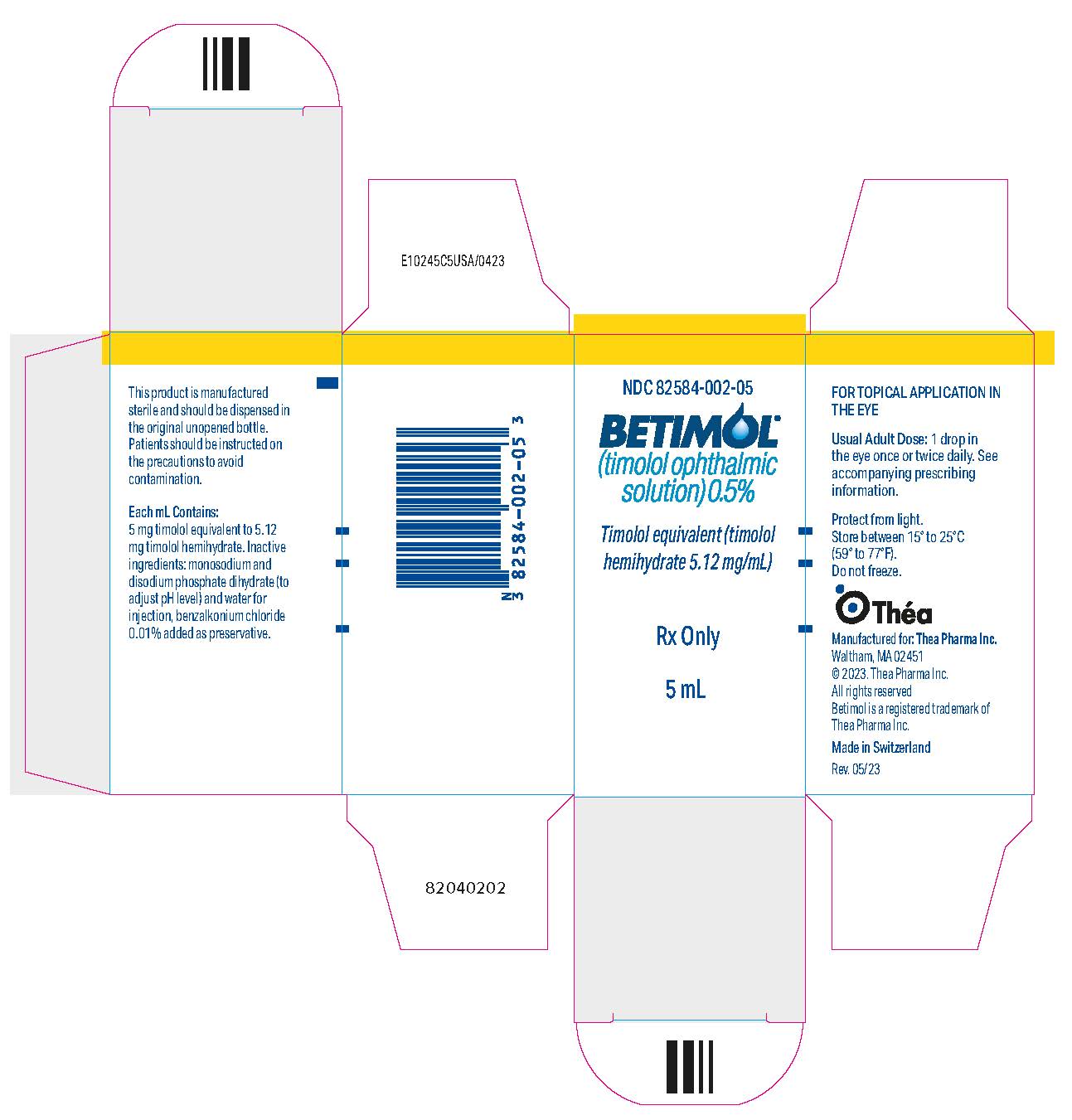
10PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
NDC 82584-001-05
BETIMOL
Timolol equivalent (timolol
Rx Only
5 mL
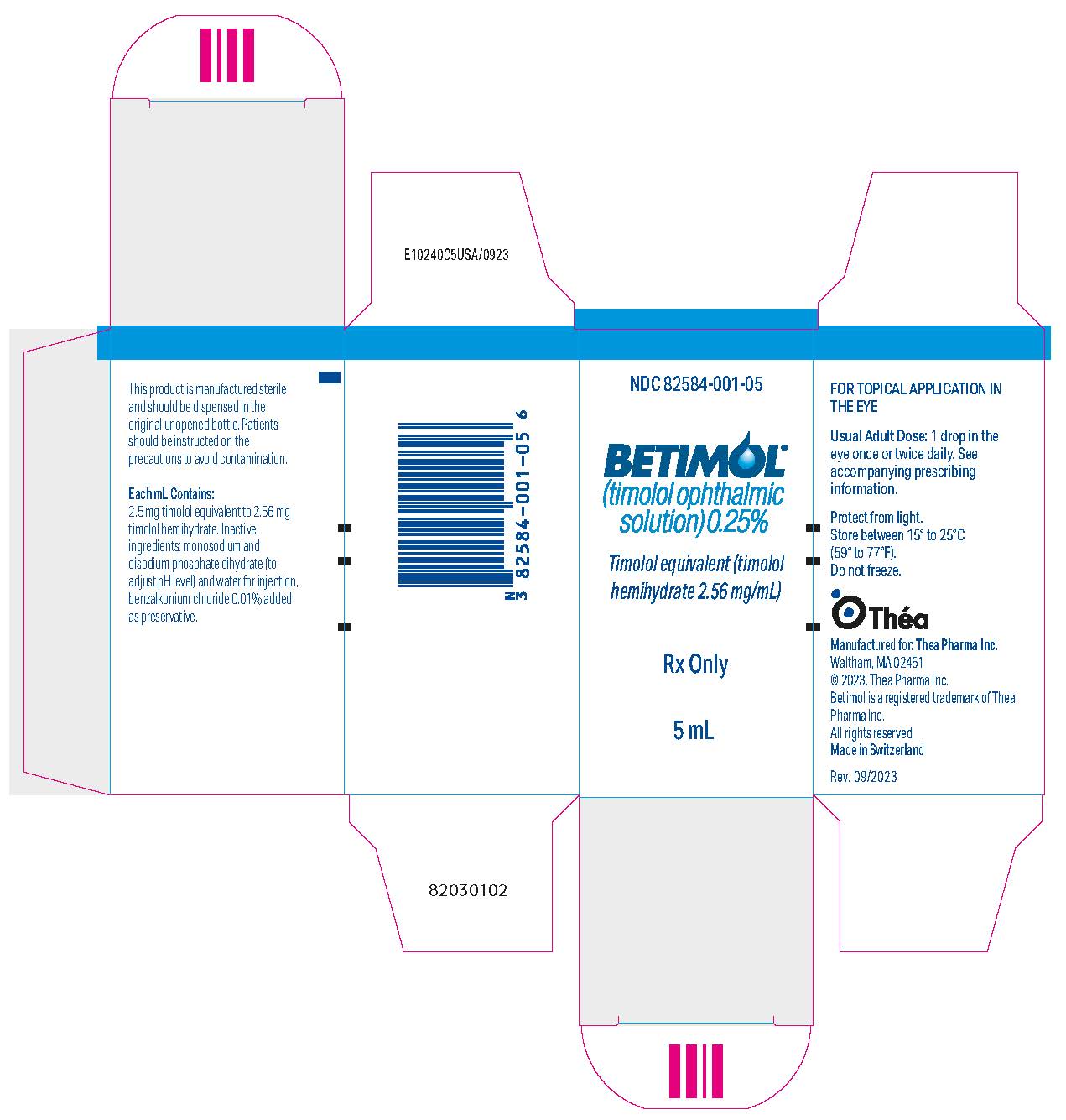
11PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton - NDC 82854-002-05
NDC 82584-002-05
BETIMOL
Timolol equivalent (timolol
Rx Only
5 mL