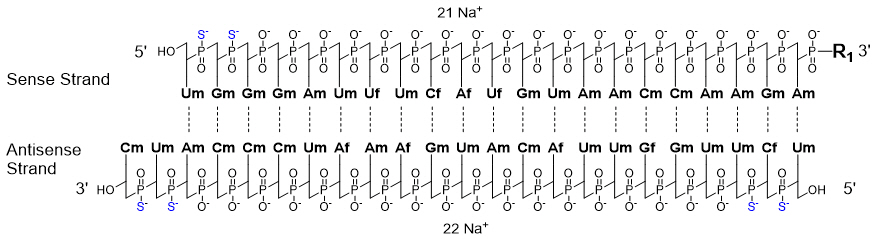
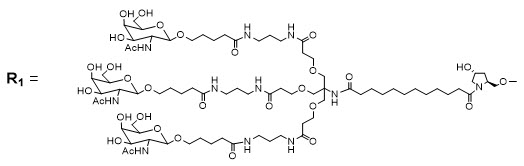
Amvuttra
What is Amvuttra (Vutrisiran)?
For people living with hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis), every movement can become a challenge. This rare, progressive disease damages nerves throughout the body, often leading to numbness, weakness, and difficulty walking. Over time, it can also affect the heart and other organs, dramatically impacting daily life. Amvuttra (vutrisiran) is a medication designed to slow this progression and help patients maintain independence and quality of life.
Amvuttra is a small interfering RNA (siRNA) therapy developed by Alnylam Pharmaceuticals. It represents a new generation of RNA-based medicines that target diseases at their genetic source. Approved by the U.S. Food and Drug Administration (FDA) in 2022, Amvuttra offers a less frequent dosing option than earlier treatments for hATTR amyloidosis, making long-term care more convenient. As a specialized therapy, it is prescribed and monitored by healthcare providers experienced in treating amyloidosis and genetic disorders of the nervous system.
What does Amvuttra do?
Amvuttra is used to treat polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) in adults. This condition results from a mutation in the TTR gene, which causes the body to produce an abnormal protein called transthyretin (TTR). These misfolded proteins form deposits, known as amyloid, that accumulate in nerves and organs, leading to nerve damage and symptoms such as pain, numbness, and loss of motor function.
The goal of Amvuttra is to reduce abnormal TTR protein production, slowing or halting further damage. In clinical studies, patients receiving vutrisiran experienced improved nerve function, better mobility, and enhanced quality of life compared with those who did not receive treatment (FDA, 2022; NIH, 2024). Some patients also reported stabilization or even reversal of neuropathy symptoms, which is particularly meaningful in a progressive disease like hATTR amyloidosis.
Amvuttra does not cure the condition, but it helps control disease progression and allows patients to preserve function and independence for longer.
How does Amvuttra work?
Amvuttra works through an innovative mechanism known as RNA interference (RNAi), a natural process that helps control gene expression. The active ingredient, vutrisiran, is a small interfering RNA molecule that specifically targets the messenger RNA (mRNA) responsible for making transthyretin (TTR) protein.
When vutrisiran enters liver cells where most TTR protein is produced, it binds to and silences the faulty TTR mRNA, preventing the production of both normal and abnormal TTR proteins. As a result, the overall levels of TTR protein in the blood decrease, leading to fewer amyloid deposits in nerves and organs.
This mechanism is clinically important because lowering TTR protein levels helps protect nerve cells from further damage. Over time, this can lead to improved nerve function, less pain, and stabilization of neurological decline.
Amvuttra represents a significant advancement in precision medicine: instead of treating symptoms alone, it targets the disease at its genetic source.
Amvuttra side effects
Most people tolerate Amvuttra well, but as with any prescription drug, side effects can occur.
Common side effects may include:
- Pain, redness, or swelling at the injection site
- Joint or muscle pain
- Fatigue or mild flu-like symptoms
- Shortness of breath (less common)
Serious side effects (rare):
- Vision changes or eye discomfort
- Signs of allergic reaction, such as rash, itching, or difficulty breathing
- Unexplained swelling or weight gain (possible fluid retention)
Because Amvuttra lowers TTR levels and TTR helps transport vitamin A, patients may develop vitamin A deficiency. For this reason, doctors recommend vitamin A supplementation during treatment to prevent vision-related side effects such as night blindness.
Patients should inform their healthcare provider about any allergies, liver problems, or prior use of similar therapies before starting treatment. Immediate medical attention should be sought if severe allergic symptoms occur.
Amvuttra dosage
Amvuttra is a subcutaneous injection given every three months by a healthcare professional, offering an infrequent dosing advantage over older treatments. Baseline evaluations, like nerve function tests and blood work, may be done before starting therapy to monitor progress.
During treatment, providers monitor Vitamin A, liver function, and neurological status. Dose adjustments are typically not needed for older adults or those with mild to moderate liver impairment, but medical supervision is crucial. Patients should only self-inject if trained and authorized by their healthcare provider.
Does Amvuttra have a generic version?
As of 2025, Amvuttra (vutrisiran) does not have a generic version available in the United States or internationally. Because it is a newly approved biologic medication with a proprietary RNA interference design, it remains under patent protection. However, international versions may exist in other markets.
Patients may receive financial aid from Alnylam Pharmaceuticals or specialty pharmacies. Future generic or biosimilar versions must meet FDA safety, purity, and efficacy standards. Patients should discuss options with their provider or insurer.
Conclusion
Amvuttra (vutrisiran) offers a breakthrough approach for people living with hereditary transthyretin-mediated amyloidosis, a rare and challenging disease. By targeting the genetic source of abnormal protein production, it helps slow or halt disease progression, protect nerve function, and improve quality of life.
Amvuttra offers patients with hATTR amyloidosis a chance to maintain mobility, reduce symptoms, and regain control over their daily lives, though it isn’t a cure. Close collaboration with a specialist, adherence to treatment, vitamin A supplements, and regular checkups are essential for managing this rare condition, helping patients live more fully.
References
- U.S. Food and Drug Administration (FDA). (2022). Amvuttra (vutrisiran) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Vutrisiran injection: Uses, side effects, and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Vutrisiran injection: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). RNA interference therapies for hereditary transthyretin amyloidosis. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The goal of this clinical trial is to investigate whether new imaging techniques can help us to better understand the cardiac amyloidosis. The disease can be slowed down with various medications (e.g., tafamidis, acoramidis, or vutrisiran). However, treatment is not effective in all patients-in about one-third of cases, the disease continues to progress. So far, we know little about the exact caus...
Summary: The purpose of this study is to: * Describe epidemiological and clinical characteristics, natural history and real-world clinical management of ATTR amyloidosis patients * Characterize the safety and effectiveness of patisiran and vutrisiran as part of routine clinical practice in the real-world clinical setting * Describe disease emergence/progression in pre-symptomatic carriers of a known diseas...
Summary: ATTRv amyloidosis is a systemic disease with two clinical forms, neurological and cardiological, which are sometimes combined (so-called mixed forms). Patisiran and vutrisiran have shown protective effects on the progression of neurological damage. The effects of Patisiran or vutrisiran on the heart remain incompletely understood. The aim of this study is to better understand the morphological and...
Related Latest Advances
Brand Information
- Reduced Serum Vitamin A Levels and Recommended Supplementation