Tresiba
What is Tresiba (Degludec)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to evaluate how well LY4057996 is tolerated and what side effects may occur in healthy participants and participants with Type 1 and Type 2 Diabetes. The study drug will be administered either subcutaneously (SC) (under the skin) or intravenously (IV) (into a vein in the arm). Blood tests will be performed to check how much LY4057996 gets into the bloodstream and how l...
Summary: The main purpose of this study is to look at the amount of the study drug LY3938577 that gets into the blood stream and how long it takes the body to get rid of it. At a later stage of this study (part B and C) the blood sugar lowering effect and the duration of action of LY3938577 will be evaluated compared to Insulin Degludec. The study will also evaluate the safety and tolerability of LY3938577...
Summary: This study compares insulin icodec, taken once a week, with other basal insulins, taken once a day, in people with type 2 diabetes.The purpose of this study is to see how well the approved injectable weekly insulin icodec maintains blood sugar levels when compared to approved and available daily injectable basal insulins in people with type 2 diabetes. The participants will either be prescribed we...
Related Latest Advances
Brand Information
- Not recommended for the treatment of diabetic ketoacidosis.
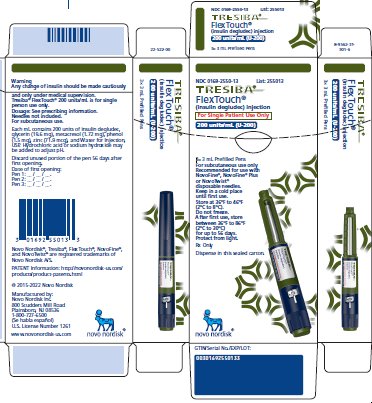
- 100 units/mL (U-100): 3 mL single-patient-use FlexTouch prefilled pen
- 100 units/mL (U-100): 10 mL multiple-dose vial
- 200 units/mL (U-200): 3 mL single-patient-use FlexTouch prefilled pen
- During episodes of hypoglycemia
- In patients with hypersensitivity to insulin degludec or any of the excipients in TRESIBA
- Hypoglycemia
- Hypoglycemia due to Medication errors
- Hypersensitivity reactions
- Hypokalemia
- 1At Week 52
- 2At Week 26
- *The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
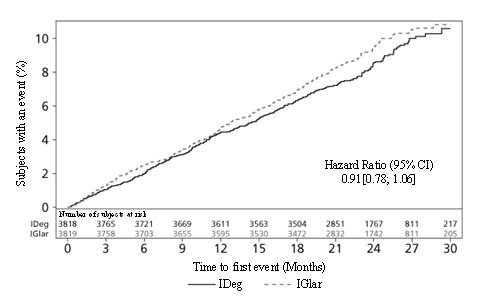
- In Study A, there were 14.8% of subjects in the TRESIBA and 11.5% Insulin glargine arms for whom data was missing at the time of the HbA
- In Study B, there were 6.3% of subjects in the TRESIBA and 9.8% Insulin detemir arms for whom data was missing at the time of the HbA
- ±The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with missing data imputed by multiple imputation carrying forward the baseline value and adding the error term, with treatment, region, sex, and age group as fixed factors, and baseline HbA1c as covariate.
- In Study J, there were 2.9% of subjects in TRESIBA and 6.3% Insulin detemir arms for whom data was missing at the 26-week HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study D, there were 20.6% of subjects in the TRESIBA and 22.2% Insulin glargine arms for whom data was missing at the time of the HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study E, there were 12.3% of subjects in the TRESIBA and 12.7% Insulin glargine arms for whom data was missing at the time of the HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study F, there were 10% of subjects in the TRESIBA and 6.8% Insulin glargine arms for whom data was missing at the time of the HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study G, there were 11.4% subjects for TRESIBA (both same time and alternating times) and 11.7% Insulin glargine arms for whom data was missing at the time of the HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study H, there were 16.1% of subjects in the TRESIBA and 14.5% Insulin glargine arms for whom data was missing at the time of the HbA
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study I, there were 20.9% of subjects in the TRESIBA and 22.5% Sitagliptin arms for whom data was missing at the time of the HbA
- 1p <0.001; 1-sided p-value evaluated at 2.5% level for superiority
(insulin degludec)
- TRESIBA is a man-made insulin that is used to control high blood sugar in adults and children who are 1 year of age and older with diabetes mellitus.
- TRESIBA is not for people with diabetic ketoacidosis (increased ketones in the blood or urine).
- It is not known if TRESIBA is safe and effective in children under 1 year of age.
- TRESIBA is available in 2 concentrations (U-100 and U-200):
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to TRESIBA or any of the ingredients in TRESIBA.
- pregnant, planning to become pregnant, or are breastfeeding.
- taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
- Read the Instructions for Use that come with your TRESIBA.
- Take TRESIBA exactly as your healthcare provider tells you to.
- Do not do any conversion of your dose. The dose counter always shows the selected dose in units. Both the 100 units/mL and 200 units/mL TRESIBA FlexTouch pens are made to deliver your insulin dose in units.
- Know the type and strength of insulin you take.
- For children who need less than 5 units of TRESIBA each day, use a TRESIBA U-100 vial.
- Adults: If you miss or are delayed in taking your dose of TRESIBA:
- If children miss a dose of TRESIBA:
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
- Never inject TRESIBA into a vein or muscle.
- Never use a syringe to remove TRESIBA from the FlexTouch pen.
- TRESIBA can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Drive or operate heavy machinery, until you know how TRESIBA affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
- Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- dizziness or light-headedness
- blurred vision
- anxiety, irritability, or mood changes
- sweating
- slurred speech
- hunger
- confusion
- shakiness
- headache
- fast heartbeat
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with TRESIBA may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure, it may get worse while you take TZDs with TRESIBA. Your healthcare provider should monitor you closely while you are taking TZDs with TRESIBA. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, tiredness, swelling of your ankles or feet and sudden weight gain. Treatment with TZDs and TRESIBA may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
- change in level of physical activity or exercise
- weight gain or loss
- increased stress
- illness
- change in diet
- serious allergic reactions (whole body reactions), reactions at the injection site, skin thickening or pits at the injection site (lipodystrophy), itching, rash, swelling of your hands and feet, and weight gain.
- trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
- a 10 mL TRESIBA vial
- a U-100 insulin syringe and needle
- 2 alcohol swabs
- 1 sharps container for throwing away used syringes and needles. See “Disposing of your used needles and syringes” at the end of these instructions.

- Do not roll or shake the TRESIBA vial. Shaking the TRESIBA vial right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin.
- The tamper-resistant cap should not be loose or damaged before the first use.
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the TRESIBA label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Check that the TRESIBA vial is not cracked or damaged.
- TRESIBA should look clear and colorless.
- Do not use TRESIBA past the expiration date printed on the label or 56 days after you start using the TRESIBA vial.
- Giving your TRESIBA injection:
- Inject your TRESIBA exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- TRESIBA can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen). Do not inject TRESIBA into your muscle.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not dilute or mix TRESIBA with any other type of insulin or solutions.
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Store unopened TRESIBA vials in the refrigerator at 36
- Do not freeze TRESIBA. Do not use TRESIBA if it has been frozen.
- Unused TRESIBA vials may be used until the expiration date printed on the label, if they are kept in the refrigerator.
- After 56 days, throw away TRESIBA vials that have been kept at room temperature (up to 86°F (30°C)).
- Store the TRESIBA vial you are currently using in the refrigerator between 36
- Keep TRESIBA away from direct heat or light.
- The TRESIBA vial you are using should be thrown away after 56 days, even if it still has insulin left in it and the expiration date has not passed.
- Keep TRESIBA vials, syringes, and needles out of the reach of children.
- Always use a new syringe and needle for each injection to help ensure sterility and prevent blocked needles.
- Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
- Novo Nordisk Inc.
- Do not share your TRESIBA FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
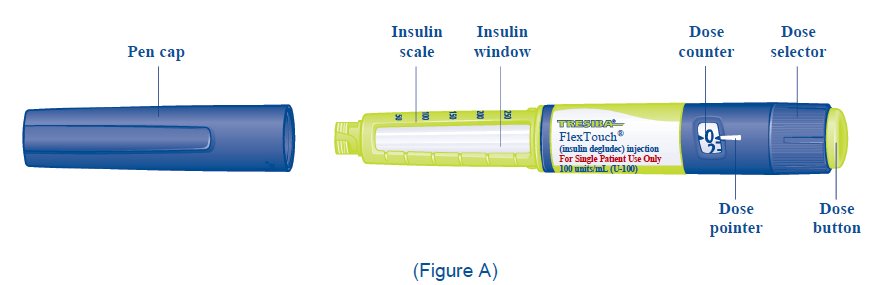
- TRESIBA FlexTouch Pen 100 units/mL (“Pen”) is a prefilled disposable, single-patient-use insulin pen containing 300 units of insulin degludec. You can inject from 1 to 80 units in a single injection. The units can be increased by 1 unit at a time.
- This Pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
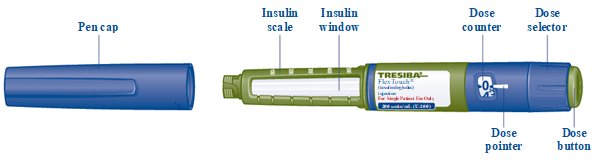
- TRESIBA FlexTouch Pen
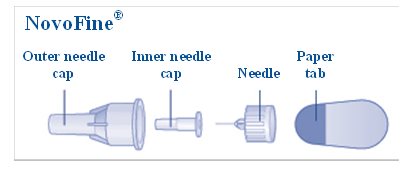
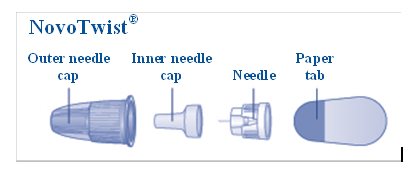
- a new NovoFine or NovoTwist needle
- alcohol swab
- a sharps container for throwing away used Pens and needles.
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the TRESIBA FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- TRESIBA should look clear and colorless.
- Do not use TRESIBA past the expiration date printed on the label or 56 days after you start using the Pen.
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
- Pull Pen cap straight off (See Figure B).
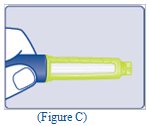
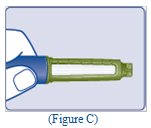
- Check the liquid in the Pen (See Figure C). TRESIBA should look clear and colorless. Do not use it if it looks cloudy or colored.
- Select a new needle.
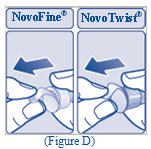
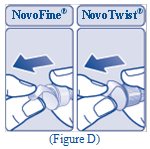
- Pull off the paper tab from the outer needle cap (See Figure D).
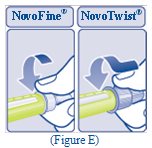
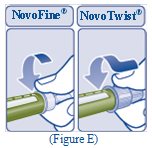
- Push the capped needle straight onto the Pen and twist the needle on until it is tight (See Figure E).
- Pull off the outer needle cap.
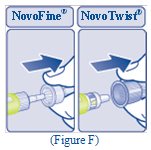
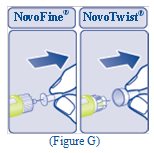
- Pull off the inner needle cap and throw it away (See Figure G).
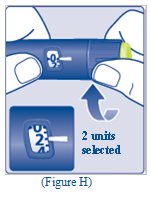
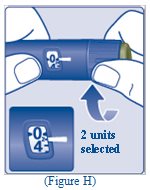
- Turn the dose selector to select 2 units (See Figure H).
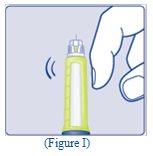
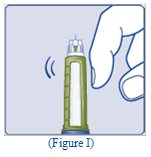
- Hold the Pen with the needle pointing up. Tap the top of the Pen gently a few times to let any air bubbles rise to the top (See Figure I).
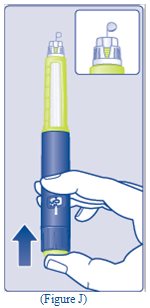
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter
- A drop of insulin should be seen at the needle tip (See Figure J).
- Turn the dose selector to select the number of units you need to inject. The dose pointer should line up
- If you select the wrong dose, you can turn the dose selector forwards or backwards to the correct dose.
- The
- The
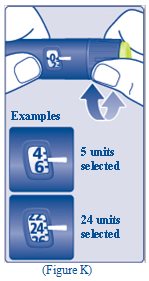
- The TRESIBA FlexTouch Pen insulin scale will show you how much insulin is left in your Pen (See Figure L).

- To see how much insulin is left in your TRESIBA FlexTouch Pen:
- Turn the dose selector until it stops. The dose counter will line up with the number of units of insulin that is left in your Pen. If the dose counter shows 80, there are at least 80 units left in your Pen.
- If the dose counter shows less than 80, the number shown in the dose counter is the number of units left in your Pen.
- Inject your TRESIBA exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- TRESIBA can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
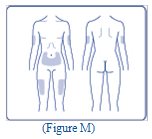
- Choose your injection site (thighs, upper arms, or abdomen) and wipe the skin with an alcohol swab (See Figure M). Let the injection site dry before you inject your dose.
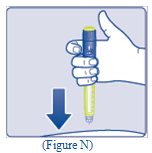
- Insert the needle into your skin (See Figure N).
- Make sure you can see the dose counter. Do not cover it with your fingers, this can stop your injection.
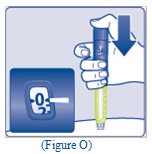
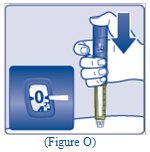
- Press and hold down the dose button until the dose counter shows “0” (See Figure O).
- The “0” must line up with the dose pointer. You may then hear or feel a click.
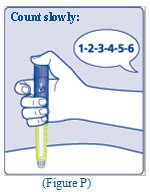
- Keep the needle in your skin after the dose counter has returned to “0” and slowly count to 6 (See Figure P).
- When the dose counter returns to “0”, you will not get your full dose until 6 seconds later.
- If the needle is removed before you count to 6, you may see a stream of insulin coming from the needle tip.
- If you see a stream of insulin coming from the needle tip you will not get your full dose. If this happens you should check your blood sugar levels more often because you may need more insulin.
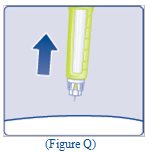
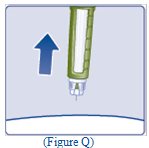
- Pull the needle out of your skin (See Figure Q).
- If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol swab.
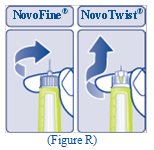
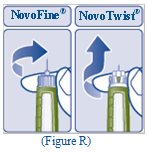
- Carefully remove the needle from the Pen and throw it away (See Figure R).
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
- If you
- Do not store the Pen with the needle attached. Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
- Replace the Pen cap by pushing it straight on (See Figure T).
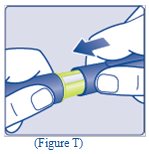
- The used TRESIBA FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Store unused TRESIBA FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze TRESIBA. Do not use TRESIBA if it has been frozen.
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
- Store the Pen you are currently using in the refrigerator between 36°F to 46°F (2°C to 8°C) or keep at room temperature up to 86°F (30°C).
- Keep TRESIBA away from heat or light.
- The TRESIBA FlexTouch Pen you are using should be thrown away after 56 days if it is refrigerated or kept at room temperature, even if it still has insulin left in it and the expiration date has not passed.
- Keep TRESIBA FlexTouch Pens and needles out of the reach of children.
- Always use a new needle for each injection.
- Do not share TRESIBA FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
Novo Nordisk Inc.

- Do not share your TRESIBA FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
- TRESIBA FlexTouch Pen 200 units/mL (“Pen”) is a prefilled disposable, single-patient-use insulin pen containing 600 units of insulin degludec. You can inject from 2 to 160 units in a single injection. The units can be increased by 2 units at a time.
- Do not use a syringe to remove insulin from your Pen. If you do, you will get too many units of insulin because the scale on most syringes is for measuring U-100 insulin doses only.
- This Pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
- TRESIBA FlexTouch Pen
- a new NovoFine or NovoTwist needle
- alcohol swab
- a sharps container for throwing away used Pens and needles.
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the TRESIBA FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- TRESIBA should look clear and colorless. Do not use TRESIBA if it is cloudy or colored.
- Do not use TRESIBA past the expiration date printed on the label or 56 days after you start using the Pen.
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
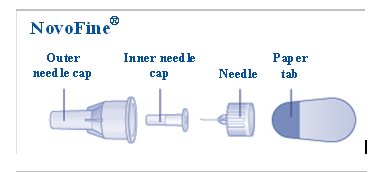
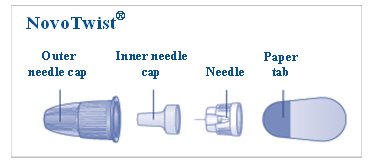
- Pull Pen cap straight off (See Figure B).
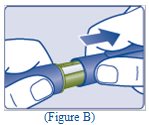
- Check the liquid in the Pen (See Figure C). TRESIBA should look clear and colorless. Do not use it if it looks cloudy or colored.
- Select a new needle.
- Pull off the paper tab from the outer needle cap (See Figure D).
- Push the capped needle straight onto the Pen and twist the needle on until it is tight (See Figure E).
- Pull off the outer needle cap. Do not throw it away (See Figure F).
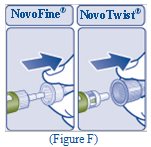
- Pull off the inner needle cap and throw it away (See Figure G).
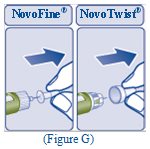
- Turn the dose selector to
- Hold the Pen with the needle pointing up. Tap the top of the Pen gently a few times to let any air bubbles rise to the top (See Figure I).
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter shows “0”. The “0” must line up with the dose pointer.
- A drop of insulin should be seen at the needle tip (See Figure J).
- If you
- If you
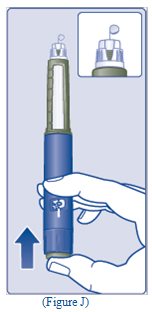
- Turn the dose selector to select the number of units you need to inject. The dose pointer should line up with your dose (See Figure K).
- If you select the wrong dose, you can turn the dose selector forwards or backwards to the correct dose.
- Each line on the dial is an even number.
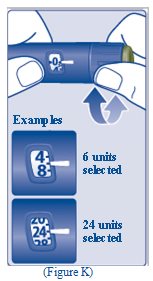
- The TRESIBA FlexTouch Pen insulin scale will show you how much insulin is left in your Pen (See Figure L).

- To see how much insulin is left in your TRESIBA FlexTouch Pen:
- o Turn the dose selector until it stops. The dose counter will line up with the number of units of insulin that is
- left in your Pen. If the dose counter shows 160, there are
- o If the dose counter shows
- in your Pen.
- Inject your TRESIBA exactly as your healthcare provider has shown you. Your healthcare provider should tell you
- TRESIBA can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
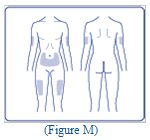
- Choose your injection site (thighs, upper arms, or abdomen) and wipe the skin with an alcohol swab (See Figure M). Let the injection site dry before you inject your dose.
- Insert the needle into your skin (See Figure N).
- Make sure you can see the dose counter.Do not cover it with your fingers, this can stop your injection.
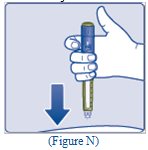
- Press and hold down the dose button until the dose counter shows “0” (See Figure O).
- o The “0” must line up with the dose pointer. You may then hear or feel a click.
- Keep the needle in your skin after the dose counter has returned to “0” and slowly count to 6 (See Figure P).
- When the dose counter returns to “0”, you will not get your full dose until 6 seconds later.
- If the needle is removed before you count to 6, you may see a stream of insulin coming from the needle tip.
- If you see a stream of insulin coming from the needle tip you will not get your full dose. If this happens you should check your blood sugar levels more often because you may need more insulin.

- Pull the needle out of your skin (See Figure Q).
- If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol swab.
- Carefully remove the needle from the Pen and throw it away (See Figure R).
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
- If you
- Do not store the Pen with the needle attached. Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
- Replace the Pen cap by pushing it straight on (See Figure T).
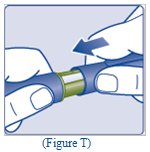
- The used TRESIBA FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Store unused TRESIBA FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze TRESIBA. Do not use TRESIBA if it has been frozen.
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
- Store the Pen you are currently using in the refrigerator between 36°F to 46°F (2°C to 8°C) or keep at room temperature up to 86°F (30°C).
- Keep TRESIBA away from heat or light.
- The TRESIBA FlexTouch Pen you are using should be thrown away after 56 days if it is refrigerated or kept at room temperature, even if it still has insulin left in it and the expiration date has not passed.
- Keep TRESIBA FlexTouch Pens and needles out of the reach of children.
- Always use a new needle for each injection.
- Do not share TRESIBA FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.

- NDC 0169-2662-11 List 266211
- (insulin degludec) injection
- Use only with a U-100 syringe.
- Rx Only
- NDC 0169-2660-15 List: 266015
- NDC 0169-2550-13 List: 255013
disposable needles.