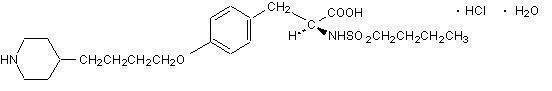
Two large-scale clinical studies established the efficacy of AGGRASTAT in the treatment of patients with NSTE-ACS (unstable angina/non-ST elevation MI). The two studies examined AGGRASTAT alone and added to heparin, prior to and after percutaneous coronary revascularization (if indicated) (PRISM-PLUS) and in comparison to heparin in a similar population (PRISM). These trials are discussed in detail below.
PRISM-PLUS (Platelet Receptor Inhibition for Ischemic Syndrome Management — Patients Limited by Unstable Signs and Symptoms)
In the double-blind PRISM-PLUS trial, 1570 patients with documented NSTE-ACS within 12 hours of entry into the study were randomized to AGGRASTAT (30 minute initial infusion of 0.4 mcg/kg/min followed by a maintenance infusion of 0.10 mcg/kg/min) in combination with heparin (bolus of 5,000 U followed by an infusion of 1,000 U/h titrated to maintain an activated partial thromboplastin time (APTT) of approximately 2 times control) or to heparin alone. All patients received concomitant aspirin unless contraindicated. Patients who were medically managed or who underwent revascularization procedures were studied. Patients underwent 48 hours of medical stabilization on study drug therapy, and they were to undergo angiography before 96 hours (and, if indicated, angioplasty/atherectomy, while continuing on AGGRASTAT and heparin for 12-24 hours after the procedure). AGGRASTAT and heparin could be continued for up to 108 hours. Exclusions included contraindications to anticoagulation, decompensated heart failure, platelet count <150,000/mm
A third group of patients was initially randomized to AGGRASTAT alone (no heparin). This arm was stopped when the group was found, at an interim look, to have greater mortality than the other two groups.
The primary endpoint of the study was a composite of refractory ischemia, new MI and death within 7 days. There was a 32% risk reduction in the overall composite primary endpoint. The components of the composite were examined separately and the results are shown in Table 6. Note that the sum of the individual components may be greater than the composite (if a patient experiences multiple component events only one event counts towards the composite).
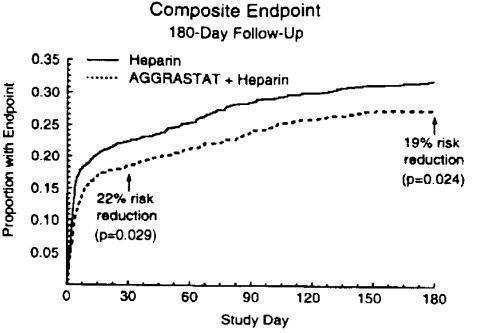
The benefit seen at 7 days was maintained over time. The risk reduction in the composite endpoint at 30 days and 6 months is shown in the Kaplan-Meier curve below.
Figure 1. Time to first event of death, new MI, or refractory ischemia in PRISM-PLUS
An analysis of the results by sex suggests that women who are medically managed or who undergo subsequent percutaneous transluminal coronary angioplasty (PTCA)/atherectomy may receive less benefit from AGGRASTAT (95% confidence limits for relative risk of 0.61-1.74) than do men (0.43-0.89) (p=0.11). This difference may be a true treatment difference, the effect of other differences in these subgroups, or a chance occurrence.
Approximately 90% of patients in the PRISM-PLUS study underwent coronary angiography and 30% underwent angioplasty/atherectomy during the first 30 days of the study. The majority of these patients continued on study drug throughout these procedures. AGGRASTAT was continued for 12-24 hours (average 15 hours) after angioplasty/atherectomy. The effects of AGGRASTAT at Day 30 did not appear to differ among sub-populations that did or did not receive PTCA or CABG, both prior to and after the procedure.
PRISM (Platelet Receptor Inhibition for Ischemic Syndrome Management)
In the PRISM study, a randomized, parallel, double-blind study, 3232 patients with NSTE-ACS intended to be managed without coronary intervention were randomized to AGGRASTAT (initial dose of 0.6 mcg/kg/min for 30 minutes followed by 0.15 mcg/kg/min for 47.5 hours) or heparin (5000-unit intravenous bolus followed by an infusion of 1000 U/h for 48 hours). The mean age of the population was 62 years; 32% of the population was female and 25% had non-ST elevation MI on presentation. Thirty percent had no ECG evidence of cardiac ischemia. Exclusion criteria were similar to PRISM-PLUS. The primary endpoint was the composite endpoint of refractory ischemia, MI or death at the end of the 48-hour drug infusion. The results are shown in Table 7.
In the PRISM study, no adverse effect of AGGRASTAT on mortality at either 7 or 30 days was detected. This result is different from that in the PRISM-PLUS study, where the arm that included AGGRASTAT without heparin (N=345) was dropped at an interim analysis by the Data Safety Monitoring Committee for increased mortality at 7 days.