Cardura
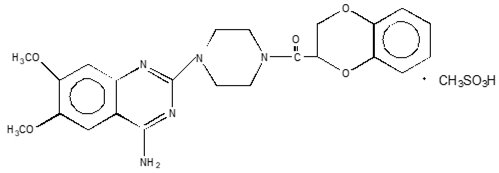
What is Cardura (Doxazosin)?
Approved To Treat
Related Clinical Trials
Summary: The goal of this research is to replicate findings previously conducted in a pilot trial and to understand, mechanistically, the role of stress in the development of AUD pharmacotherapies that target noradrenergic blockade.
Summary: Multiple sclerosis (MS) is the leading non-traumatic cause of severe acquired disability in young people. The disease is defined by relapses, which can affect all neurological functions depending on the location of the new inflammatory lesion(s). The disease can thus manifest itself through bladder and bowel disorders (BWS), which affect approximately 80% of MS patients in all stages. Lower urinar...
Summary: Cerebral small vessel disease (SVD) describes a set of pathologies affecting the smallest blood vessels in the brain. SVD contributes to up to a fifth of ischemic and hemorrhagic strokes en is the main vascular cause of dementia. On MRI, SVD is marked by different types of lesions, including white matter abnormalities, and small infarcts and hemorrhages. Recent studies indicate that SVD develops s...
Related Latest Advances
Brand Information
- have or have had low blood pressure, especially after taking another medicine. Signs of low blood pressure are fainting, dizziness, and lightheadedness.
- plan to have cataract surgery
- have stomach problems
- have prostate cancer
- have liver problems
- have heart problems
- a medicine to treat an infection
- a medicine to treat HIV
- a medicine to treat depression
- a medicine to treat erectile dysfunction (ED)
- Take CARDURA XL exactly as your healthcare provider tells you to take it.
- Take CARDURA XL 1 time each day with your breakfast.
- Take CARDURA XL tablets whole.
- CARDURA XL tablets have an outside shell that helps to release the medicine inside over time. You may see the empty CARDURA XL tablet in your stool (bowel movement). This is normal.
- If you take too much CARDURA XL, call your healthcare provider or go to the nearest hospital emergency room right away.
- a sudden drop in blood pressure (postural hypotension). Postural hypotension can happen at any time while you take CARDURA XL. The chance of having postural hypotension is higher a few hours after you take CARDURA XL or if you start taking a higher dose.
Symptoms of postural hypotension may happen after you stand up from a lying or sitting position and may include:- dizziness or
- fainting or
- feeling light-headed
- You should get up slowly from a chair or bed until you know how CARDURA XL will affect you. If you begin to feel dizzy or lightheaded, sit or lie down with your legs and feet up. If your symptoms do not get better call your healthcare provider.
- intraoperative floppy iris syndrome (IFIS). IFIS can happen during eye surgery to people who are taking or have taken alpha-blockers such as CARDURA XL. If you have an eye surgery for a clouding of the eye (cataract) planned, tell your eye doctor that you are using CARDURA XL or have taken an alpha-blocker before.
- blockage of the digestive tract. There have been rare reports of symptoms of blockage in the digestive tract in patients with prior history of narrowing or blockage of the digestive tract. These symptoms are severe and persistent abdominal pain/cramping/bloating. If you know you have a narrowing of your digestive tract and you experience these symptoms, contact your healthcare provider.
- prolonged erection of the penis. Extremely rarely, CARDURA XL and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
- tiredness
- headache
- low blood pressure
- dizziness
- Store CARDURA XL at 59°F to 86°F (15°C to 30°C).
[see USP Controlled Room
Temperature].
See accompanying
prescribing information.

[see USP Controlled Room
Temperature].
See accompanying
prescribing information.