Viread
What is Viread (Tenofovir Disoproxil)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Background: People with HIV take drugs to keep the amount of virus in their body low. One type of these drugs, called integrase strand transfer inhibitors (INSTIs), can cause weight gain over time. Weight gain can cause diabetes, heart disease, and other serious issues. Researchers want to understand how INSTIs cause weight changes.
Summary: Recent studies have identified an association between Alzheimer's Disease (AD) and an expansion of DNA content in the brain (prefrontal cortex). This additional DNA content appears to be derived from reverse transcriptase (RT) activity that incorporates genomic cDNAs (gencDNAs) into chromosomes, resulting in multiple copies of full length and shorter cDNAs involving many genes - including the caus...
Summary: The goal of this observational, multicenter , real-world study is to evaluate the long-term outcomes of different antiviral therapies in adults with chronic hepatitis B (CHB). The main questions it aims to answer are: What is the 5-year incidence of hepatocellular carcinoma (HCC) under various treatment regimens? How do rates of HBsAg seroclearance, decompensated cirrhosis, liver fibrosis progress...
Related Latest Advances
Brand Information
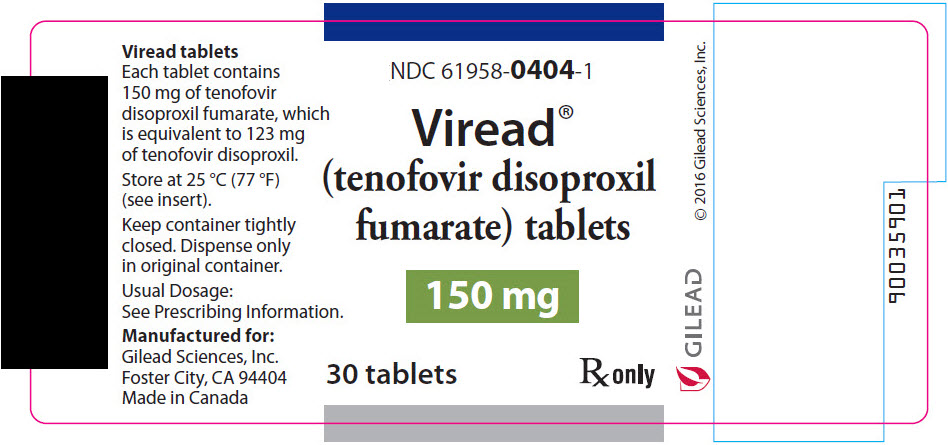
- 150 mg Tablets: 150 mg of tenofovir disoproxil fumarate (TDF) (equivalent to 123 mg of tenofovir disoproxil): triangle shaped, white, film coated, debossed with "GSI" on one side and with "150" on the other side.
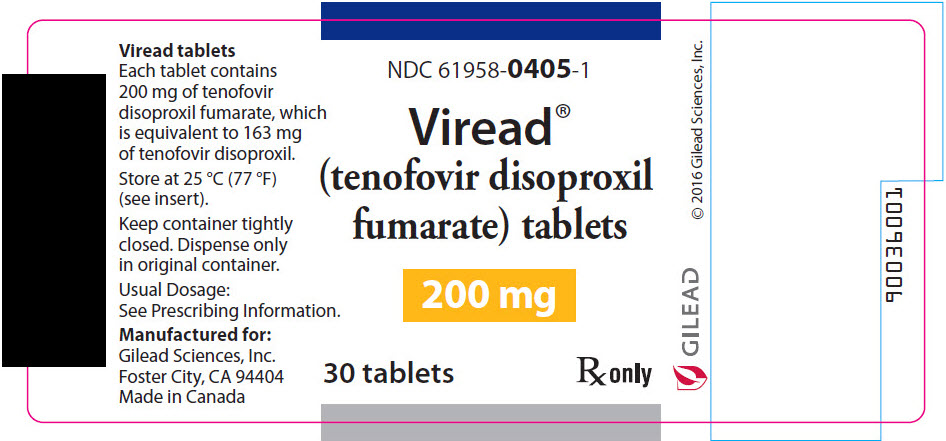
- 200 mg Tablets: 200 mg of TDF (equivalent to 163 mg of tenofovir disoproxil): round shaped, white, film coated, debossed with "GSI" on one side and with "200" on the other side.
- 250 mg Tablets: 250 mg of TDF (equivalent to 204 mg of tenofovir disoproxil): capsule shaped, white, film coated, debossed with "GSI" on one side and with "250" on the other side.
- 300 mg Tablets: 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil): almond shaped, light blue, film coated, debossed with "GILEAD" and "4331" on one side and with "300" on the other side.
- Oral Powder: white, taste-masked, coated granules containing 40 mg of TDF (equivalent to 33 mg of tenofovir disoproxil) per level scoop. Each level scoop contains 1 gram of oral powder.
- Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection
- New Onset or Worsening Renal Impairment
- Immune Reconstitution Syndrome
- Bone Loss and Mineralization Defects
- Lactic Acidosis/Severe Hepatomegaly with Steatosis
allergic reaction, including angioedema
lactic acidosis, hypokalemia, hypophosphatemia
dyspnea
pancreatitis, increased amylase, abdominal pain
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)
rash
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
asthenia
- 150 mg of TDF (equivalent to 123 mg of tenofovir disoproxil): tablets are triangle-shaped, white, film-coated, and debossed with "GSI" on one side and with "150" on the other side. (NDC 61958-0404-1)
- 200 mg of TDF (equivalent to 163 mg of tenofovir disoproxil): tablets are round-shaped, white, film-coated, and debossed with "GSI" on one side and with "200" on the other side. (NDC 61958-0405-1)
- 250 mg of TDF (equivalent to 204 mg of tenofovir disoproxil): tablets are capsule-shaped, white, film-coated and debossed with "GSI" on one side and with "250" on the other side. (NDC 61958-0406-1)
- 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil): tablets are almond-shaped, light-blue, film-coated, and debossed with "GILEAD" and "4331" on one side and with "300" on the other side. (NDC 61958-0401-1)
- VIREAD powder comes in a box that has a bottle of VIREAD and a dosing scoop (see
- Only use the dosing scoop to measure VIREAD powder.
- Only mix VIREAD powder with soft foods that can be swallowed without chewing. Examples of soft foods you can use are: applesauce, baby food, or yogurt.
- Do not mix VIREAD powder with liquid. The powder may float to the top even after stirring.
- Give the entire dose right away after mixing to avoid a bad taste.
- Wash your hands well with soap and water, and dry them.
- Measure ¼ to ½ cup of soft food such as applesauce, baby food, or yogurt into a cup or bowl.
- To open a new bottle of powder, press down on the bottle lid and turn to remove (see picture on the top of the bottle cap). Peel off the foil.
- Measure the number of scoops prescribed by your healthcare provider.
- Sprinkle the VIREAD powder on the soft food. Stir with a spoon until well mixed.
- Close the bottle of VIREAD tightly.
- Wash and dry the dosing scoop.
- Store VIREAD powder at room temperature between 68 °F to 77 °F (20 °C to 25 °C).
- Keep VIREAD powder in the original container.
- Keep the bottle tightly closed.
- Do not use VIREAD powder if the seal over the bottle opening is broken or missing.