Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
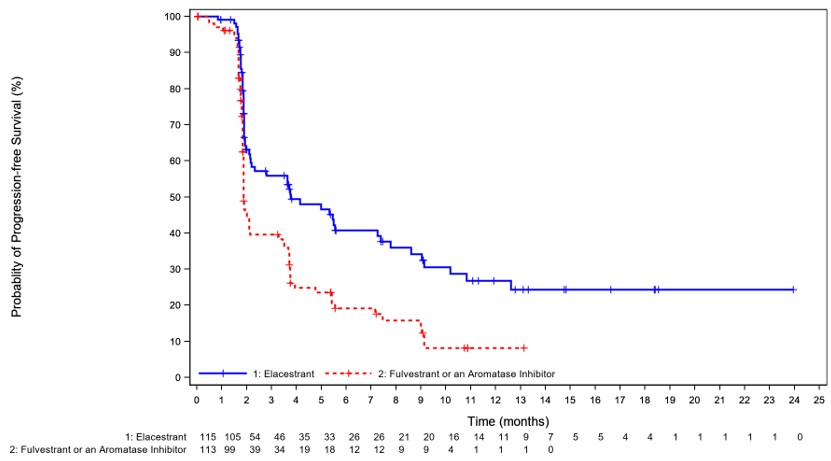
The safety of ORSERDU was evaluated in 467 patients with ER+/HER2- advanced breast cancer following CDK4/6 inhibitor therapy in EMERALD, a randomized, open-label, multicenter study
Serious adverse reactions occurred in 12% of patients who received ORSERDU. Serious adverse reactions in >1% of patients who received ORSERDU were musculoskeletal pain (1.7%) and nausea (1.3%). Fatal adverse reactions occurred in 1.7% of patients who received ORSERDU, including cardiac arrest, septic shock, diverticulitis, and unknown cause (one patient each).
Permanent discontinuation of ORSERDU due to an adverse reaction occurred in 6% of patients. Adverse reactions which resulted in permanent discontinuation of ORSERDU in >1% of patients were musculoskeletal pain (1.7%) and nausea (1.3%).
Dosage interruptions of ORSERDU due to an adverse reaction occurred in 15% of patients. Adverse reactions which resulted in dosage interruption of ORSERDU in >1% of patients were nausea (3.4%), musculoskeletal pain (1.7%), and increased ALT (1.3%).
Dosage reductions of ORSERDU due to an adverse reaction occurred in 3% of patients. Adverse reactions which required dosage reductions of ORSERDU in >1% of patients were nausea (1.7%).
The most common (≥10%) adverse reactions, including laboratory abnormalities, of ORSERDU were musculoskeletal pain, nausea, increased cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flush, and dyspepsia.
Table 3 summarizes the adverse reactions in EMERALD. Table 3: Adverse Reactions (>10%) in Patients with ER-positive, HER2-negative, Advanced or Metastatic Breast Cancer Who Received ORSERDU in EMERALD
Clinically relevant adverse reactions in < 10% of patients who received ORSERDU included rash, insomnia, dyspnea, cough, dizziness, stomatitis and gastroesophageal reflux disease.
Table 4 summarizes the laboratory abnormalities in EMERALD. Table 4: Select Laboratory Abnormalities (>10%) That Worsened from Baseline in Patients with ER-positive, HER2-negative, Advanced or Metastatic Breast Cancer Who Received ORSERDU in EMERALD