Lomaira
What is Lomaira (Phentermine)?
Weight loss can be a difficult and emotional journey, especially when lifestyle changes alone don’t bring the desired results. For some individuals struggling with obesity or weight-related health problems, certain prescription medications can provide an extra level of support. One such medication is Phentermine, a well-established prescription drug that has been used for decades to assist with weight management under medical supervision.
Phentermine belongs to a class of drugs known as sympathomimetic amines, which act as appetite suppressants. It is typically prescribed as part of a short-term treatment plan alongside a reduced-calorie diet, exercise, and behavioral modifications.
What does Phentermine do?
Phentermine is primarily used for short-term weight reduction in adults with obesity (BMI ≥30) or those who are overweight (BMI ≥27) with associated health conditions such as high blood pressure, diabetes or high cholesterol.
By reducing appetite and increasing energy expenditure, Phentermine helps patients eat less and make healthier food choices while staying motivated in their weight-loss journey. Clinical studies and real-world use have shown that when combined with lifestyle interventions, Phentermine can lead to significant short-term weight loss, typically over a few weeks to months (Mayo Clinic, 2024; MedlinePlus, 2024).
Beyond the physical benefits, patients often report feeling more in control of their eating habits, which can improve self-confidence and overall quality of life.
How does Phentermine work?
Phentermine works by stimulating the central nervous system (CNS) specifically targeting regions of the brain that control appetite. It increases the release of certain neurotransmitters, including norepinephrine, dopamine and serotonin. These chemicals help suppress hunger signals and increase alertness and energy levels.
This mechanism mimics the body’s natural “fight or flight” response, which temporarily reduces appetite. Clinically, this helps patients feel full longer and consume fewer calories throughout the day.
The mechanism matters because sustained calorie reduction, even modest, can trigger meaningful weight loss when combined with consistent lifestyle changes. However, since Phentermine acts on the CNS, it should always be taken under close medical supervision to minimize potential side effects or dependency risks (FDA, 2024).
Phentermine side effects
Like all prescription medications, Phentermine can cause side effects. Most are mild and improve as the body adjusts, but some may require medical attention.
Common side effects include:
- Dry mouth
- Trouble sleeping (insomnia)
- Restlessness or nervousness
- Increased heart rate or blood pressure
- Constipation
Less common or serious side effects:
- Chest pain or shortness of breath
- Severe headache or dizziness
- Mood changes (e.g., irritability, agitation)
- Allergic reactions such as rash or swelling
Patients with a history of heart disease, uncontrolled high blood pressure, hyperthyroidism, glaucoma or substance misuse should generally avoid Phentermine. It is also not recommended during pregnancy or breastfeeding.
If symptoms such as chest pain, irregular heartbeat, or severe mood changes occur, immediate medical attention is necessary (NIH, 2024).
Phentermine dosage
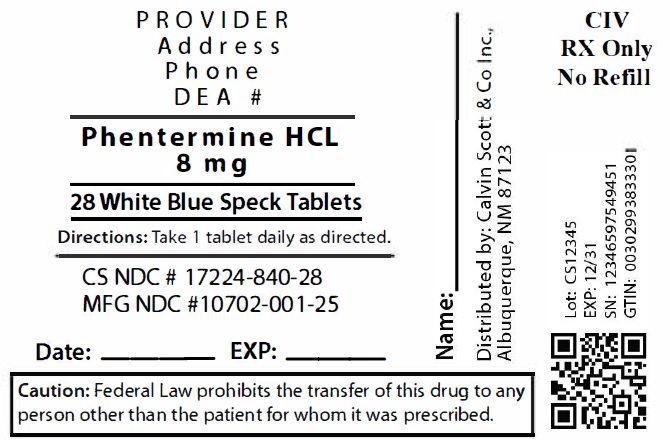
Phentermine is available in several oral forms, including tablets, capsules, and orally disintegrating tablets. It is typically taken once daily, preferably in the morning before breakfast or a few hours after, to avoid insomnia.
Because Phentermine is a short-term medication (usually up to 12 weeks), healthcare providers will monitor patients’ blood pressure, heart rate and weight progress during treatment. In some cases, additional lab tests may be ordered to ensure safety and effectiveness.
Older adults or patients with kidney, liver, or cardiovascular disease may require closer monitoring or an alternative approach. It is crucial to follow the doctor’s instructions carefully and never increase the dose independently, as higher doses do not enhance effectiveness and may raise health risks (FDA, 2024).
Does Phentermine have a generic version?
Yes, Phentermine is available in generic form, which is FDA-approved and considered equally effective and safe as brand-name products. Common brand names include Adipex-P and Lomaira.
Generic Phentermine offers the same active ingredient and therapeutic benefits at a typically lower cost, making it more accessible for patients. However, as with all medications, the choice between brand-name and generic versions should be made in consultation with a healthcare provider or pharmacist.
Conclusion
Phentermine can be a valuable tool for adults struggling with obesity when used responsibly under medical supervision. By reducing appetite and supporting healthy lifestyle changes, it helps patients achieve meaningful weight loss that can improve both physical health and emotional well-being.
While it is not a magic solution, when paired with sustained diet and exercise habits, Phentermine can play a key role in long-term weight management. Patients should maintain open communication with their healthcare providers to monitor progress, manage side effects, and adjust treatment as needed.
Phentermine is safe and effective when prescribed and monitored by a qualified healthcare provider. With medical guidance and lifestyle commitment, it can serve as a stepping stone toward a healthier and more confident future.
References
- U.S. Food and Drug Administration (FDA). (2024). Phentermine Label Information. https://www.fda.gov/
- Mayo Clinic. (2024). Phentermine for Weight Loss. https://www.mayoclinic.org/
- MedlinePlus. (2024). Phentermine: Drug Information. https://medlineplus.gov/
- National Institutes of Health (NIH). (2024). Obesity and Pharmacologic Management. https://www.nih.gov/
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information