Brand Name
Banzel
Generic Name
Rufinamide
View Brand Information FDA approval date: November 14, 2008
Form: Tablet, Suspension
What is Banzel (Rufinamide)?
Rufinamide tablets USP are indicated for adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome in pediatric patients 1 year of age and older, and in adults Rufinamide tablets USP are indicated for adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome in pediatric patients 1 year of age and older and in adults.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Banzel (rufinamide)
1INDICATIONS AND USAGE
BANZEL is indicated for adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome in pediatric patients 1 year of age and older and in adults.
2DOSAGE FORMS AND STRENGTHS
Film-coated Tablets: 200 mg (pink) and 400 mg (pink). Tablets are scored on both sides.
Oral Suspension: 40 mg/mL. White to off-white opaque liquid.
3CONTRAINDICATIONS
BANZEL is contraindicated in patients with Familial Short QT syndrome
4ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Suicidal Behavior and Ideation
- Central Nervous System Reactions
- QT Shortening
- Multi-Organ Hypersensitivity/Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
- Leukopenia
4.1Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adult and Pediatric Patients ages 3 to 17 years of age
In the pooled, double-blind, adjunctive therapy studies in adult and pediatric patients ages 3 to 17 years of age, the most common (≥10%) adverse reactions in BANZEL-treated patients, in all doses studied (200 to 3200 mg per day) with a higher frequency than in patients on placebo were: headache, dizziness, fatigue, somnolence, and nausea.
Table 2 lists adverse reactions that occurred in at least 3% of pediatric patients (ages 3 to less than 17 years) with epilepsy treated with BANZEL in controlled adjunctive studies and were numerically more common in patients treated with BANZEL than in patients on placebo.
At the target dose of 45 mg/kg per day for adjunctive therapy in pediatric patients (ages 3 to less than 17 years), the most common (≥3%) adverse reactions with an incidence greater than in placebo for BANZEL were somnolence, vomiting, and headache.
Table 3 lists adverse reactions that occurred in at least 3% of adult patients with epilepsy treated with BANZEL (up to 3200 mg per day) in adjunctive controlled studies and were numerically more common in patients treated with BANZEL than in patients on placebo. In these studies, either BANZEL or placebo was added to the current AED therapy.
At all doses studied of up to 3200 mg per day given as adjunctive therapy in adults, the most common (≥ 3%) adverse reactions, and with the greatest increase in incidence compared to placebo, for BANZEL were dizziness, fatigue, nausea, diplopia, vision blurred, and ataxia.
Discontinuation in Controlled Clinical Studies
In controlled, double-blind, adjunctive clinical studies, 9% of pediatric and adult patients receiving BANZEL as adjunctive therapy and 4% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of BANZEL (>1%) used as adjunctive therapy were generally similar in adults and pediatric patients.
In pediatric patients (ages 4 to less than 17 years) double-blind adjunctive clinical studies, 8% of patients receiving BANZEL as adjunctive therapy (at the recommended dose of 45 mg/kg per day) and 2% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of BANZEL (>1%) used as adjunctive therapy are presented in Table 4.
In adult double-blind, adjunctive clinical studies, 10% of patients receiving BANZEL as adjunctive therapy (at doses up to 3200 mg per day) and 6% receiving placebo discontinued as a result of an adverse reaction. The adverse reactions most commonly leading to discontinuation of BANZEL (>1%) used as adjunctive therapy are presented in Table 5.
Pediatric Patients ages 1to less than 4 years
In a multicenter, parallel group, open-label study comparing BANZEL (45 mg/kg per day) adjunctive treatment (n=25) to the adjunctive treatment with an AED of the investigator’s choice (n=11) in pediatric patients (1 year to less than 4 years of age) with inadequately controlled Lennox-Gastaut Syndrome, the adverse reaction profile was generally similar to that observed in adults and pediatric patients 4 years of age and older treated with BANZEL. Adverse reactions that occurred in at least 2 (8%) BANZEL-treated patients and with a higher frequency than in the AED comparator group were: vomiting (24%), somnolence (16%), bronchitis (12%), constipation (12%), cough (12%), decreased appetite (12%), rash (12%), otitis media (8%), pneumonia (8%), decreased weight (8%), gastroenteritis (8%), nasal congestion (8%), and pneumonia aspiration (8%).
Other Adverse Reactions Observed During Clinical Trials
BANZEL has been administered to 1978 individuals during all epilepsy clinical trials (placebo-controlled and open-label). Adverse reactions occurring during these studies were recorded by the investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of patients having adverse reactions, these events were grouped into standardized categories using the MedDRA dictionary. Adverse events occurring at least three times and considered possibly related to treatment are included in the System Organ Class listings below. Terms not included in the listings are those already included in the tables above, those too general to be informative, those related to procedures, and terms describing events common in the population. Some events occurring fewer than 3 times are also included based on their medical significance. Because the reports include events observed in open-label, uncontrolled observations, the role of BANZEL in their causation cannot be reliably determined.
Events are classified by body system and listed in order of decreasing frequency as follows:
Blood and Lymphatic System Disorders: Frequent:anemia. Infrequent:lymphadenopathy, leukopenia, neutropenia, iron deficiency anemia, thrombocytopenia.
Cardiac Disorders: Infrequent: bundle branch block right, atrioventricular block first degree.
Metabolic and Nutritional Disorders: Frequent: decreased appetite, increased appetite.
Renal and Urinary Disorders: Frequent: pollakiuria. Infrequent:urinary incontinence, dysuria, hematuria, nephrolithiasis, polyuria, enuresis, nocturia, incontinence.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of BANZEL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: Stevens-Johnson syndrome and other serious skin rashes with mucosal involvement.
5OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Certified Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
One overdose of 7200 mg per day BANZEL was reported in an adult during the clinical trials. The overdose was associated with no major signs or symptoms, no medical intervention was required, and the patient continued in the study at the target dose.
Treatment or Management of Overdose: There is no specific antidote for overdose with BANZEL. If clinically indicated, elimination of unabsorbed drug should be attempted by induction of emesis or gastric lavage. Usual precautions should be observed to maintain the airway. General supportive care of the patient is indicated including monitoring of vital signs and observation of the clinical status of the patient.
Hemodialysis: Standard hemodialysis procedures may result in limited clearance of rufinamide. Although there is no experience to date in treating overdose with hemodialysis, the procedure may be considered when indicated by the patient’s clinical state.
6DESCRIPTION
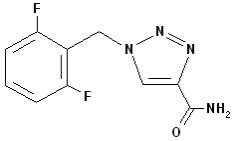
BANZEL (rufinamide) is a triazole derivative structurally unrelated to currently marketed antiepileptic drugs (AEDs). Rufinamide has the chemical name 1-[(2,6-difluorophenyl)methyl]-1
BANZEL is available for oral administration in film-coated tablets, scored on both sides, containing 200 and 400 mg of rufinamide. Inactive ingredients are colloidal silicon dioxide, corn starch crosscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulphate. The film coating contains hypromellose, iron oxide red, polyethylene glycol, talc, and titanium dioxide.
BANZEL is also available for oral administration as a liquid containing rufinamide at a concentration of 40 mg/mL. Inactive ingredients include microcrystalline cellulose and carboxymethylcellulose sodium, hydroxyethylcellulose, anhydrous citric acid, simethicone emulsion 30%, poloxamer 188, methylparaben, propylparaben, propylene glycol, potassium sorbate, noncrystallizing sorbitol solution 70%, and an orange flavor.
7CLINICAL STUDIES
Adult and Pediatric Patients ages 4 years and older
The effectiveness of BANZEL as adjunctive treatment for the seizures associated with Lennox-Gastaut Syndrome (LGS) in adult and pediatric patients ages 4 years and older was established in a single multicenter, double-blind, placebo-controlled, randomized, parallel-group study (N=138). Male and female patients (between 4 and 30 years of age) were included if they had a diagnosis of inadequately controlled seizures associated with LGS (including both atypical absence seizures and drop attacks) and were being treated with 1 to 3 concomitant stable dose AEDs. Each patient must have had at least 90 seizures in the month prior to study entry. After completing a 4-week Baseline Phase on stable therapy, patients were randomized to have BANZEL or placebo added to their ongoing therapy during the 12 -week Double-blind Phase. The Double-blind Phase consisted of 2 periods: the Titration Period (1 to 2 weeks) and the Maintenance Period (10 weeks). During the Titration Period, the dose was increased to a target dosage of approximately 45 mg/kg per day (3200 mg in adults of
The primary efficacy variables were:
- The percent change in total seizure frequency per 28 days;
- The percent change in tonic-atonic (drop attacks) seizure frequency per 28 days;
- Seizure severity from the Parent/Guardian Global Evaluation of the patient’s condition. This was a 7-point assessment performed at the end of the Double-blind Phase. A score of +3 indicated that the patient’s seizure severity was very much improved, a score of 0 that the seizure severity was unchanged, and a score of -3 that the seizure severity was very much worse.
The results of the three primary endpoints are shown in Table 7 below.
Pediatric Patients ages 1 to less than 4 years
The effectiveness of BANZEL as adjunctive treatment for the seizures associated with Lennox-Gastaut Syndrome in pediatric patients ages 1 year to less than 4 years was established based on a single multi-center, open-label, active-controlled, randomized, pharmacokinetic bridging study. The pharmacokinetic profile of BANZEL is not significantly affected by age either as a continuous covariate (1 to 35 years) or as a categorical covariate (age categories: 1 to less than 4 years and 4 years of age and older), after body weight is taken into consideration.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Administration Information
- Advise patients to take BANZEL with food
- Advise patients who are prescribed the oral suspension to shake the bottle vigorously before every administration and to use the adaptor and oral dosing syringe
Suicidal Thinking and Behavior
Inform patients, their caregivers, and families that antiepileptic drugs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers
Central Nervous System Reactions
Inform patients about the potential for somnolence or dizziness and advise them not to drive or operate machinery until they have gained sufficient experience on BANZEL to gauge whether it adversely affects their mental and/or motor performance
Multi-Organ Hypersensitivity Reactions
Advise patients to notify their physician if they experience a rash associated with fever
Drug Interactions
- Inform female patients of childbearing age that the concurrent use of BANZEL with hormonal contraceptives may render this method of contraception less effective. Recommend patients use additional non-hormonal forms of contraception when using BANZEL
- Inform patients that alcohol in combination with BANZEL may cause additive central nervous system effects.
Pregnancy
Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. To enroll, patients can call the toll free number 1-888-233-2334
Breast-feeding
Advise patients to notify their physician if they are breast-feeding or intend to breast-feed