Brand Name
Elrexfio
Generic Name
Elranatamab-Bcmm
View Brand Information FDA approval date: August 15, 2023
Form: Injection
What is Elrexfio (Elranatamab-Bcmm)?
ELREXFIO is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. This indication is approved under accelerated approval based on response rate and durability of response. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial. ELREXFIO is a bispecific B-cell maturation antigen -directed CD3 T‑cell engager indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. This indication is approved under accelerated approval based on response rate and durability of response. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Study of Elranatamab Management With Outpatient and Intermittent Dosing in Relapsed/Refractory Multiple Myeloma
Summary: A phase II study of single agent elranatamab in patients with relapsed and/or refractory multiple myeloma (MM) who have previously received at least three classes of therapeutic agents and are refractory to the last line of treatment. The primary objective of this study is to improve the tolerability and safety of elranatamab in patients with relapsed and/or refractory multiple myeloma by evaluati...
Related Latest Advances
Brand Information
Elrexfio (elranatamab-bcmm)
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITY including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
- Cytokine Release Syndrome (CRS), including life-threatening or fatal reactions, can occur in patients receiving ELREXFIO. Initiate treatment with ELREXFIO step-up dosing schedule to reduce the risk of CRS. Withhold ELREXFIO until CRS resolves or permanently discontinue based on severity Dosage and Administration (2.2, .
- Neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), and serious and life-threatening reactions, can occur in patients receiving ELREXFIO. Monitor patients for signs and symptoms of neurologic toxicity, including ICANS, during treatment. Withhold ELREXFIO until the neurologic toxicity resolves or permanently discontinue based on severity Dosage and Administration (2.5), .
- Because of the risk of CRS and neurologic toxicity, including ICANS, ELREXFIO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called ELREXFIO REMS
1INDICATIONS AND USAGE
ELREXFIO is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
This indication is approved under accelerated approval based on response rate and durability of response
2DOSAGE FORMS AND STRENGTHS
ELREXFIO injection is a clear to slightly opalescent, and colorless to pale brown liquid solution available as:
- 76 mg/1.9 mL (40 mg/mL) in a single-dose vial
- 44 mg/1.1 mL (40 mg/mL) in a single-dose vial
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling:
- Cytokine Release Syndrome
- Neurologic Toxicity, Including ICANS
- Infections
- Neutropenia
- Hepatotoxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed/Refractory Multiple Myeloma
MagnetisMM-3
The safety of ELREXFIO was evaluated in MagnetisMM-3
The median age of patients who received ELREXFIO was 68 years (range: 36 to 88 years); 48% were female; 61% were White, 10% were Hispanic/Latino, 9% were Asian, and 6% were Black or African American.
Serious adverse reactions occurred in 68% of patients who received ELREXFIO at the recommended dosing schedule. Serious adverse reactions in >2% of patients included pneumonia (25%), sepsis (13%), CRS (13%), upper respiratory tract infection (4.4%), acute kidney injury (3.8%), urinary tract infection (3.3%), COVID-19 (3.3%), encephalopathy (3.3%), pyrexia (2.2%), and febrile neutropenia (2.2%). Fatal adverse reactions occurred in 10% of patients including pneumonia (3.3%), sepsis (2.7%), acute respiratory distress syndrome (0.5%), cardio-respiratory arrest (0.5%), cardiogenic shock (0.5%), cardiopulmonary failure (0.5%), COVID-19 (0.5%), failure to thrive (0.5%), and pulmonary embolism (0.5%).
Permanent discontinuations of ELREXFIO due to an adverse reaction occurred in 17% of patients. Adverse reactions which resulted in permanent discontinuation of ELREXFIO in >2% of patients included septic shock (2.2%).
Dosage interruptions of ELREXFIO due to an adverse reaction occurred in 73% of patients. Adverse reactions which resulted in dose interruptions of ELREXFIO in >5% of patients included neutropenia, pneumonia, COVID-19, upper respiratory tract infection, thrombocytopenia, and anemia.
The most common adverse reactions (≥20%) were CRS, fatigue, injection site reaction, diarrhea, upper respiratory tract infection, musculoskeletal pain, pneumonia, decreased appetite, rash, cough, nausea, and pyrexia. The most common Grade 3 to 4 laboratory abnormalities (≥30%) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased white blood cells, and decreased platelets.
Table 8 summarizes adverse reactions in MagnetisMM-3.
Clinically relevant adverse reactions in <10% of patients who received ELREXFIO included ICANS, febrile neutropenia, Guillain-Barré syndrome, abdominal pain, acute kidney injury, COVID-19, cardiac failure, congestion, thrombosis and cytomegalovirus infection.
Table 9 summarizes laboratory abnormalities in MagnetisMM-3.
5DRUG INTERACTIONS
For certain CYP substrates, minimal changes in the concentration may lead to serious adverse reactions. Monitor for toxicity or drug concentrations of such CYP substrates when co-administered with ELREXFIO.
ELREXFIO causes release of cytokines
6DESCRIPTION
Elranatamab-bcmm is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager. It is a bispecific, humanized immunoglobulin 2-alanine (IgG2Δa) kappa antibody derived from two monoclonal antibodies (mAbs), an anti-BCMA mAb and an anti-CD3 mAb. Each of these mAbs contributes one distinct heavy (H) chain and one distinct light (L) chain to the bispecific elranatamab-bcmm. The resulting 4-chain bispecific antibody is covalently linked via five inter-chain disulfide bonds. Elranatamab-bcmm is produced using two recombinant Chinese hamster ovary (CHO) cell lines, one that contains the DNA encoding the sequence for anti-BCMA monoclonal antibody (mAb) and one that contains the sequence for anti-CD3 mAb, which are grown separately in suspension culture using chemically-defined (CD), animal-derived component-free (ACF) media. The molecular weight of elranatamab-bcmm is approximately 148.5 kDa.
ELREXFIO
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ELREXFIO
- One 76 mg/1.9 mL (40 mg/mL) single-dose vial in a carton. NDC: 0069-4494-02
- One 44 mg/1.1 mL (40 mg/mL) single-dose vial in a carton. NDC: 0069-2522-02
ELREXFIO is supplied in a single-dose glass vial sealed with a rubber stopper (not made of natural rubber latex) and an aluminum seal with a flip-off cap.
Storage and Handling
Store refrigerated at 2 °C to 8 °C (36 °F to 46 °F) in the original carton until time of use to protect from light.
Do not freeze or shake the vial or carton.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome (CRS)
Discuss the signs and symptoms associated with CRS, including fever, hypoxia, chills, hypotension, tachycardia, and elevated liver enzymes. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of CRS. Advise patients that they will be hospitalized for 48 hours after administration of the first step-up dose, and for 24 hours after administration of the second step-up dose
Neurologic Toxicity, Including Immune Effector Cell-associated Neurotoxicity Syndrome (ICANS)
Discuss the signs and symptoms associated with neurologic toxicity, including ICANS, including headache, encephalopathy, motor dysfunction, sensory neuropathy, and Guillain-Barré Syndrome. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of neurologic toxicity. Advise patients to refrain from driving or operating heavy or potentially dangerous machinery for 48 hours after completing each of the 2 step-up doses and the first treatment dose within the ELREXFIO step-up dosing schedule and in the event of new onset of any neurological toxicity symptoms until symptoms resolve
ELREXFIO REMS
ELREXFIO is available only through a restricted program called ELREXFIO REMS. Inform patients that they will be given an ELREXFIO Patient Wallet Card that they should carry with them at all times and show to all of their healthcare providers. This card describes signs and symptoms of CRS and neurologic toxicity, including ICANS which, if experienced, should prompt the patient to immediately seek medical attention
Infections
Discuss the signs and symptoms of infection
Neutropenia
Discuss the signs and symptoms associated with neutropenia and febrile neutropenia
Hepatotoxicity
Advise patients that liver enzyme elevations may occur and that they should report symptoms that may indicate liver toxicity, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant. Advise females of reproductive potential to use effective contraception during treatment with ELREXFIO and for 4 months after the last dose
Lactation
Advise women not to breastfeed during treatment with ELREXFIO and for 4 months after the last dose
Manufactured by:
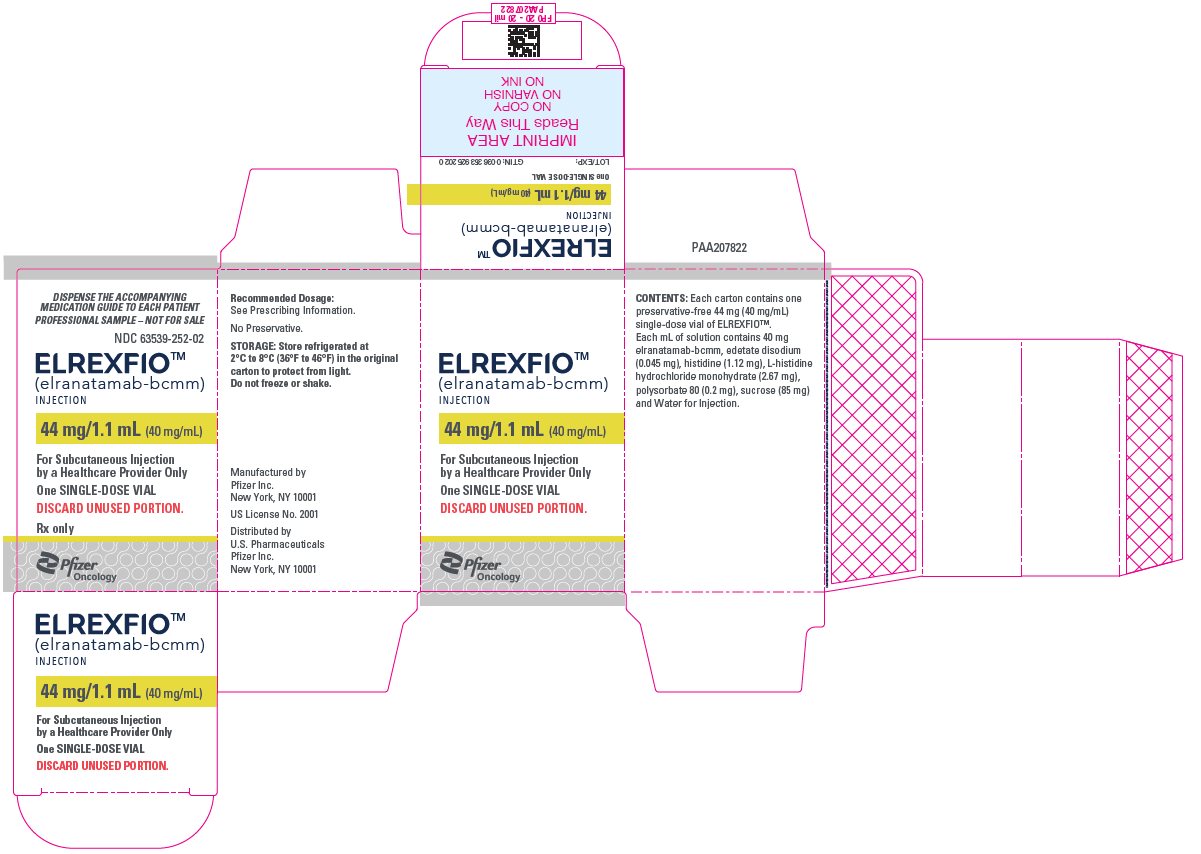
9PRINCIPAL DISPLAY PANEL – 44 mg/1.1 mL Vial
DISPENSE THE ACCOMPANYING
NDC 63539-252-01
ELREXFIO
44 mg/1.1 mL (40 mg/mL)
One SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Pfizer

10PRINCIPAL DISPLAY PANEL – 44 mg/1.1 mL Vial Carton
DISPENSE THE ACCOMPANYING
NDC 63539-252-02
ELREXFIO
44 mg/1.1 mL (40 mg/mL)
For Subcutaneous Injection
One SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Rx only
PfizerOncology