Brand Name
Altuviiio
Generic Name
Fc-VWF-XTEN
View Brand Information FDA approval date: February 22, 2023
Form: Kit
What is Altuviiio (Fc-VWF-XTEN)?
ALTUVIIIO [antihemophilic factor , Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A for: Routine prophylaxis to reduce the frequency of bleeding episodes On-demand treatment and control of bleeding episodes Perioperative management of bleeding ALTUVIIIO [antihemophilic factor , Fc-VWF-XTEN fusion protein-ehtl] is a recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A for: Routine prophylaxis to reduce the frequency of bleeding episodes On-demand treatment & control of bleeding episodes Perioperative management of bleeding Limitation of Use: ALTUVIIIO is not indicated for the treatment of von Willebrand disease. Limitation of Use ALTUVIIIO is not indicated for the treatment of von Willebrand disease.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Prospective, Observational Study of the Impact of Efanesoctocog Alfa (ALTUVIIIO®) on Goal Attainment and Physical Activity in People With Moderate or Severe Hemophilia A
Summary: This is a Phase 4, multi-center, observational study conducted in patients aged 12 to 50 years with moderate or severe hemophilia A who are newly starting prophylaxis with efa in the US and Japan. This study aims to enroll 35 patients.
Related Latest Advances
Brand Information
ALTUVIIIO (Antihemophilic Factor (Recombinant), Fc-VWF-XTEN Fusion Protein-ehtl)
1INDICATIONS AND USAGE
ALTUVIIIO is indicated for use in adults and pediatric patients with hemophilia A (congenital factor VIII deficiency) for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
2DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1Dose
- Each ALTUVIIIO vial label states the Factor VIII potency in international units (IU). One IU corresponds to the Factor VIII activity contained in one milliliter of normal human plasma, as defined by the current World Health Organization (WHO) international standard for Factor VIII concentrate.
- Potency assignment for ALTUVIIIO is determined using an activated partial thromboplastin time (aPTT)-based one-stage clotting assay. It is recommended to use a validated one-stage clotting assay to measure ALTUVIIIO Factor VIII activity in plasma. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold
For the dose of 50 IU/kg, the expected
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL).
2.2Preparation and Reconstitution
- Use aseptic technique and a flat work surface during the reconstitution procedure.
- Allow the ALTUVIIIO vial, containing the white to off-white lyophilized powder, and the prefilled diluent syringe to reach room temperature before use.
- Remove the plastic cap from the ALTUVIIIO vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
- Completely remove the backing from the vial adapter package by peeling back the lid.
- Keep the vial on a flat surface. Hold the vial with one hand and using the other hand, place the vial adapter in its package over the vial. The spike should be placed directly above the center of the rubber stopper. Push the vial adapter straight down until the spike on the vial adapter punctures the center of the vial stopper and is fully inserted.
- Lift the package cover away from the vial adapter and throw away the cover.
- Only use the prefilled diluent syringe provided to reconstitute the powdered medicine. Hold the plunger rod by the circular disk. Place the tip of the plunger rod into the end of the prefilled diluent syringe. Turn the plunger rod to the right until it is firmly attached.
- With one hand, hold the prefilled diluent syringe directly under the cap with the cap pointing up. Make sure you are holding the prefilled diluent syringe by the ridged part directly under the cap.
- With your other hand, grasp the cap and bend it at a 90 degree angle until it snaps off. After the cap snaps off, you will see the glass tip of the prefilled diluent syringe.
- Be sure the vial is sitting on a flat surface. Insert the tip of the prefilled diluent syringe into the vial adapter opening. Turn the prefilled diluent syringe to the right until it is securely attached to the vial adapter.
- Slowly push down on the plunger rod to inject all of the liquid (diluent) from the prefilled diluent syringe into the vial. The plunger rod may rise slightly afterward. This is normal.
- With the prefilled diluent syringe still connected to the adapter, gently swirl the vial until the powder is completely dissolved. Check the solution through the vial to make sure the powder is fully dissolved. The solution should look clear and colorless to opalescent.
- Make sure the plunger rod is pressed all the way down and the diluent syringe is firmly attached to the vial adapter. Turn the vial upside-down. Slowly pull down on the plunger rod to draw all the solution from the vial into the diluent syringe. Be careful not to pull the plunger rod completely out of the diluent syringe.
- Gently unscrew the diluent syringe from the vial adapter by turning it to the right. Dispose of the vial with the adapter still attached. If you are not ready to inject, put the syringe cap carefully back onto the syringe tip.
- Use the reconstituted ALTUVIIIO as soon as possible, but no later than
2.3Administration
For intravenous use only.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not administer reconstituted ALTUVIIIO in the same tubing or container with other medications.
3DOSAGE FORMS AND STRENGTHS
ALTUVIIIO is available as a white to off-white lyophilized powder for reconstitution in single-dose vials containing nominally 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU) per vial.
4CONTRAINDICATIONS
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients
5DESCRIPTION
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution for intravenous injection. The product is supplied in single-dose vials containing nominal potencies of 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU). Each vial of ALTUVIIIO is labeled with the actual Factor VIII activity content in IU. The powder for injection is reconstituted with 3 mL sterile water for injection (sWFI) supplied in a sterile prefilled syringe. The reconstituted solution should be essentially free of particles. The final product contains the excipients: arginine hydrochloride (250 mM), calcium chloride dihydrate (5 mM), histidine (10 mM), polysorbate 80 (0.05% w/v), and sucrose (5% w/v).
The active ingredient in ALTUVIIIO is a fully recombinant fusion protein comprising a single chain B-domain deleted (BDD) analogue of human FVIII covalently fused to the Fc domain of human immunoglobulin G1 (IgG1), the FVIII-binding D'D3 domain of human von Willebrand factor (VWF), and 2 XTEN polypeptides. ALTUVIIIO contains 2829 amino acids with an apparent molecular weight of 312 kDa. ALTUVIIIO is synthesized as 2 polypeptide chains which are covalently linked by 2 Fc hinge disulfide bonds. The first FVIII-XTEN-Fc polypeptide chain contains the A1A2 domain of FVIII along with 5 amino acids from B-domain (1–745 amino acids) fused to the 288-XTEN polypeptide (in place of the natural FVIII B-domain), the A3C1C2 domain of FVIII (1649–2332), and the Fc domain of human IgG1. The second VWF-XTEN-a2-Fc polypeptide chain contains the D'D3 domain of VWF (1–477 amino acids) fused to the 144-XTEN polypeptide, a thrombin cleavable acidic region 2 sequence from FVIII and the Fc domain of human IgG1. The Fc domain includes the hinge, CH
ALTUVIIIO is produced by recombinant DNA technology in a human embryonic kidney (HEK) cell line, which has been extensively characterized. ALTUVIIIO is manufactured without addition of human- or animal-derived components and purified by a combination of multiple chromatography steps, a detergent or solvent/detergent viral inactivation step, a nano filtration step for viral clearance, and ultrafiltration steps.
6CLINICAL STUDIES
The safety, efficacy, and pharmacokinetics of ALTUVIIIO were evaluated in two multicenter, prospective, open-label clinical studies, Study 1 (NCT04161495) and Study 2 (NCT04759193), as described below.
All studies evaluated the efficacy of routine prophylaxis with a weekly dose of 50 IU/kg and determined hemostatic efficacy in the treatment of bleeding episodes and during perioperative management in patients undergoing major or minor surgical procedures.
7PATIENT COUNSELING INFORMATION
Advise the patients to:
- Read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Call their healthcare provider or go to the emergency department right away if a hypersensitivity reaction occurs. Early signs of hypersensitivity reactions may include rash, hives, itching, facial swelling, tightness of the chest, and wheezing.
- Contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor VIII therapy because this may be a sign of inhibitor development.
8Patient Information
ALTUVIIIO
[antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl]
for intravenous use after reconstitution only
Single-dose vial
Please read this Patient Information carefully before using ALTUVIIIO and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I need to know about ALTUVIIIO?
Do not attempt to give yourself an injection unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for injecting ALTUVIIIO so that your treatment will work best for you.
What is ALTUVIIIO?
ALTUVIIIO is an injectable medicine that is used to control and reduce the number of bleeding episodes in people with Hemophilia A (congenital Factor VIII deficiency).
Your healthcare provider may give you ALTUVIIIO when you have surgery.
Who should not use ALTUVIIIO?
You should not use ALTUVIIIO if you had an allergic reaction to it in the past.
What should I tell my healthcare provider before using ALTUVIIIO?
Talk to your healthcare provider about:
- Any medical problems that you have or had.
- All prescription and non-prescription medicines that you take, including over-the-counter medicines, supplements or herbal medicines.
- Pregnancy or if you are planning to become pregnant. It is not known if ALTUVIIIO may harm your unborn baby.
- Breastfeeding. It is not known if ALTUVIIIO passes into the milk and if it can harm your baby.
How should I use ALTUVIIIO?
You get ALTUVIIIO as an injection into your vein. Your healthcare provider will instruct you on how to do injections on your own and may watch you give yourself the first dose of ALTUVIIIO.
Contact your healthcare provider right away if bleeding is not controlled after using ALTUVIIIO.
What are the possible side effects of ALTUVIIIO?
You can have an allergic reaction to ALTUVIIIO. Call your healthcare provider or emergency department right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash or hives.
Your body can also make antibodies called "inhibitors" against ALTUVIIIO. This can stop ALTUVIIIO from working properly. Your healthcare provider may give you blood tests to check for inhibitors.
The common side effects of ALTUVIIIO are headache and joint pain.
These are not the only possible side effects of ALTUVIIIO. Tell your healthcare provider about any side effect that bothers you or does not go away.
What are the ALTUVIIIO dosage strengths?
ALTUVIIIO comes in seven different dosage strengths with 3 mL sterile water for injection (sWFI). The actual number of international units (IU) of Factor VIII activity in the vial will be imprinted on the label and on the box. The seven different strengths are as follows:
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store ALTUVIIIO?
- Keep ALTUVIIIO in its original package.
- Protect it from light.
- Do not freeze.
- Store refrigerated 2°C to 8°C (36°F to 46°F) up to 48 months or at room temperature [not to exceed 30°C (86°F)], for a single period up to 6 months. Do not use ALTUVIIIO after the expiration date printed on the label and carton of each vial.
- When storing at room temperature:
After mixing with the diluent:
- Do not use ALTUVIIIO if the mixed solution is not clear and colorless to slightly yellowish.
- Use mixed product as soon as possible.
- You may store mixed ALTUVIIIO at room temperature, not to exceed 30°C (86°F), for up to
What else should I know about ALTUVIIIO?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use ALTUVIIIO for a condition for which it was not prescribed. Do not share ALTUVIIIO with other people, even if they have the same symptoms that you have.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
©2024 Bioverativ Therapeutics Inc. All rights reserved.
ALTUVIIIO is a registered trademark of Bioverativ Therapeutics Inc.
Revised: SEP 2024
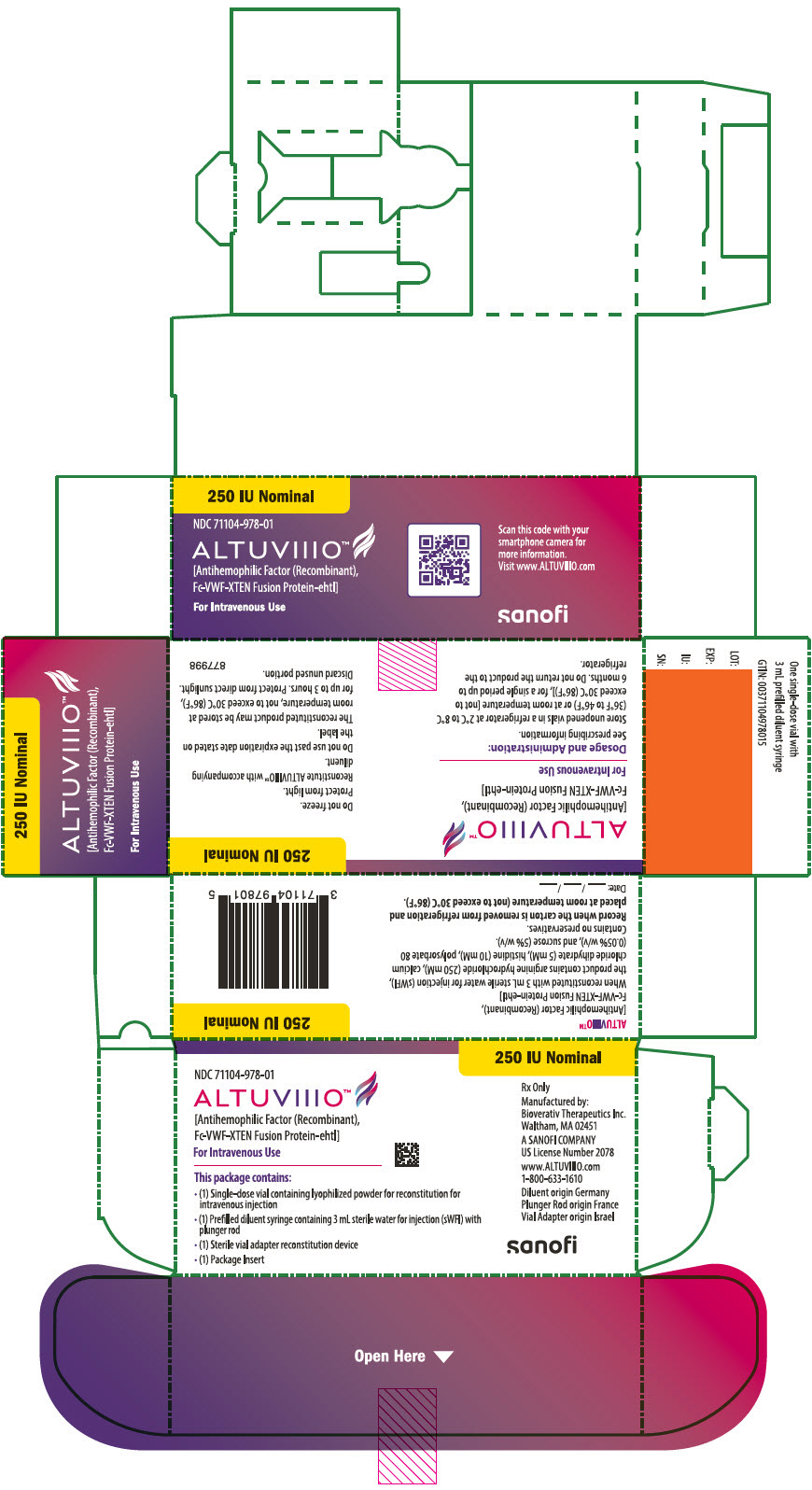
9PRINCIPAL DISPLAY PANEL - 250 IU Kit Carton
250 IU Nominal
NDC 71104-978-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi
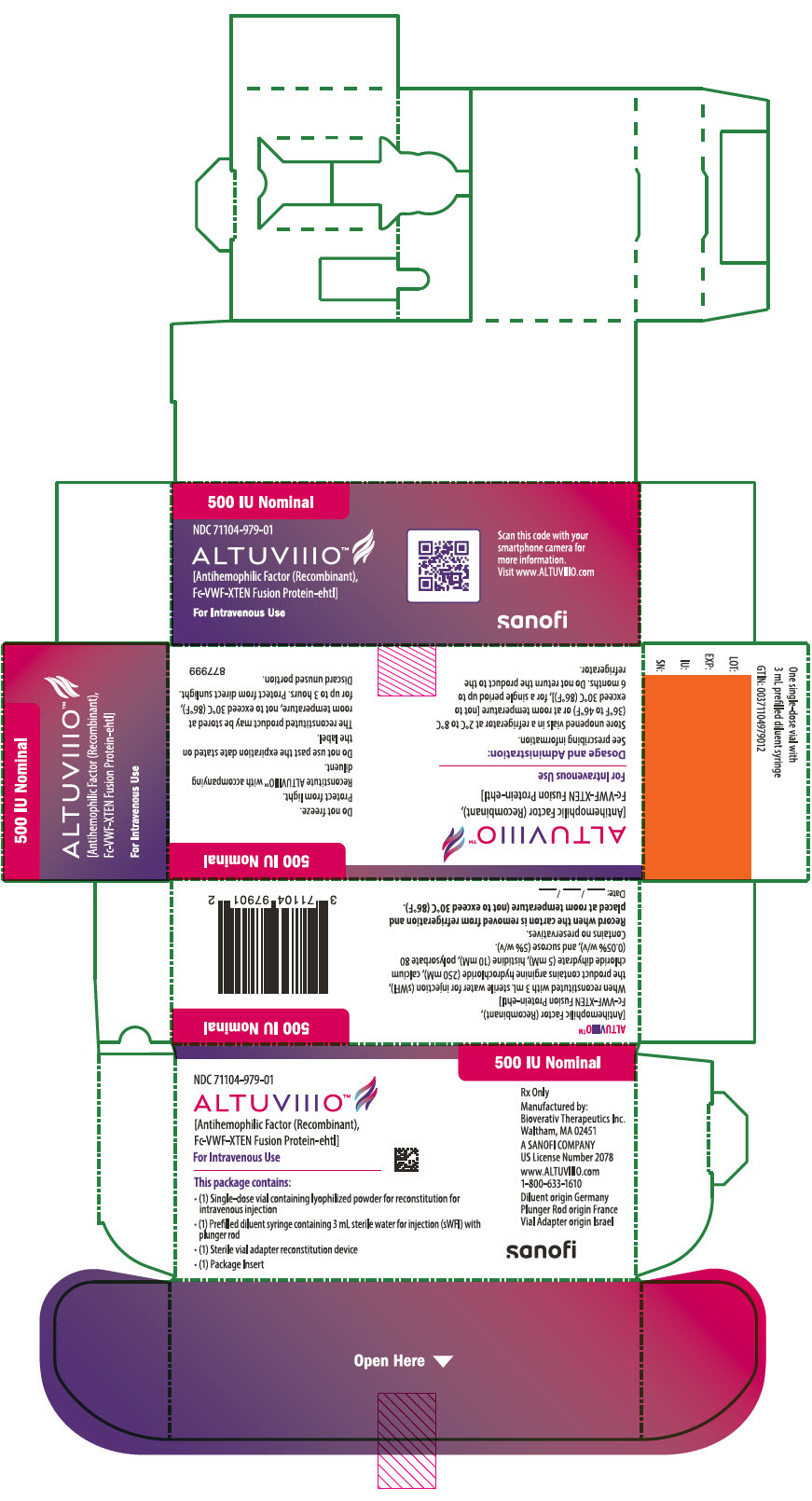
10PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton
500 IU Nominal
NDC 71104-979-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi
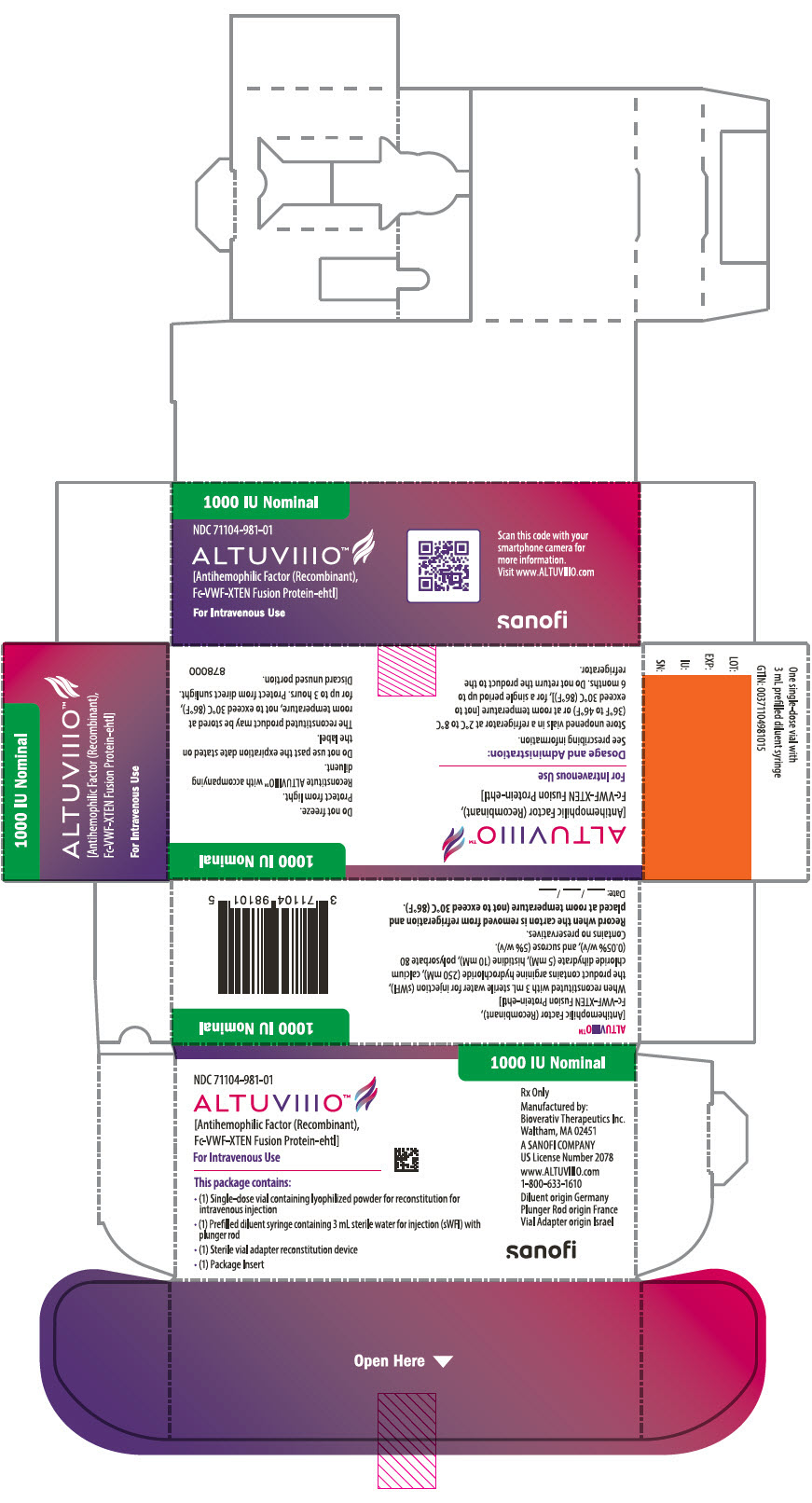
11PRINCIPAL DISPLAY PANEL - 1000 IU Kit Carton
1000 IU Nominal
NDC 71104-981-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi
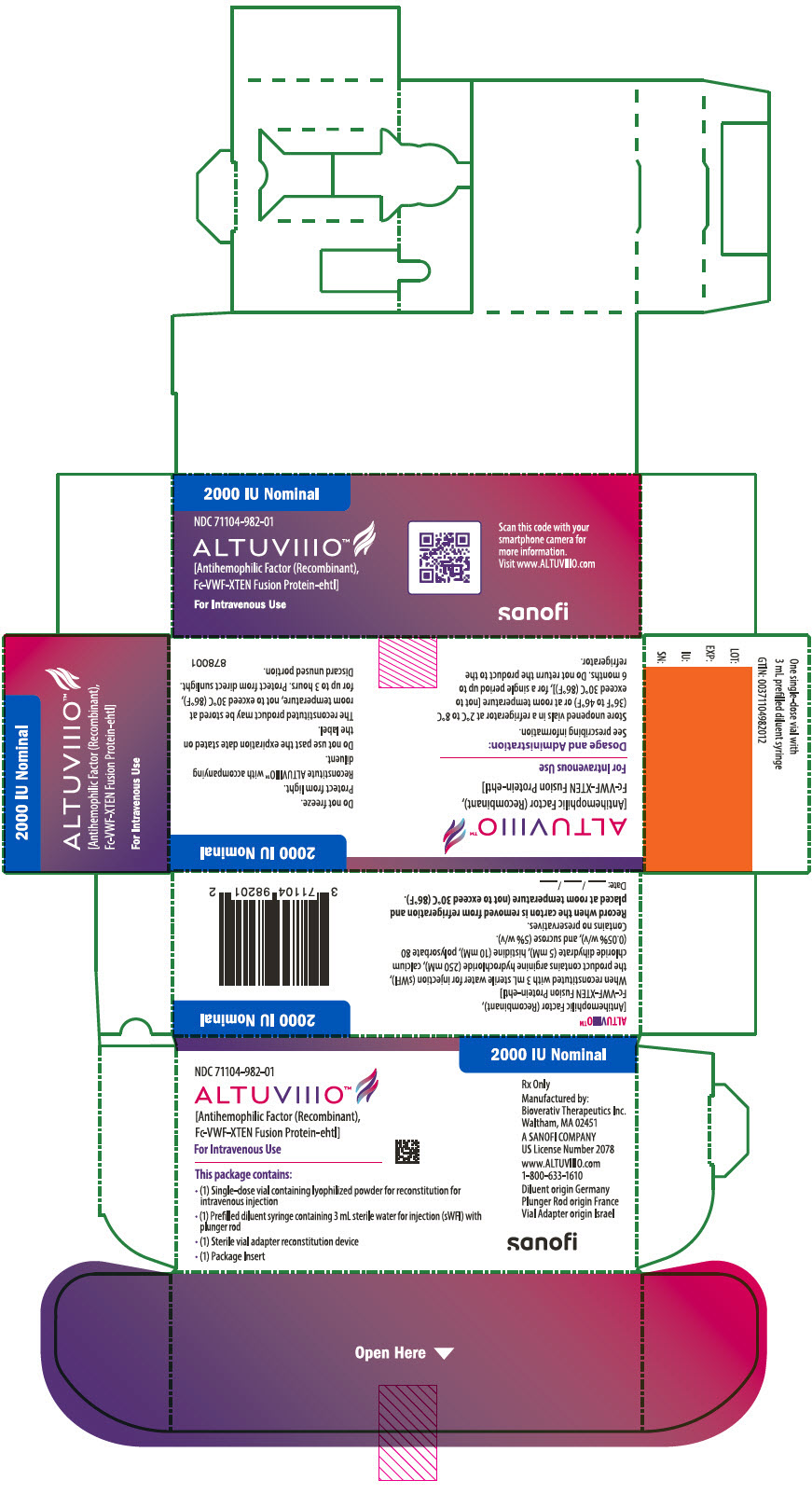
12PRINCIPAL DISPLAY PANEL - 2000 IU Kit Carton
2000 IU Nominal
NDC 71104-982-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi
13PRINCIPAL DISPLAY PANEL - 3000 IU Kit Carton
3000 IU Nominal
NDC 71104-983-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi

14PRINCIPAL DISPLAY PANEL - 4000 IU Kit Carton
4000 IU Nominal
NDC 71104-984-01
ALTUVIIIO™
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
- (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
- (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
A SANOFI COMPANY
www.ALTUVIIIO.com
Diluent origin Germany
sanofi
