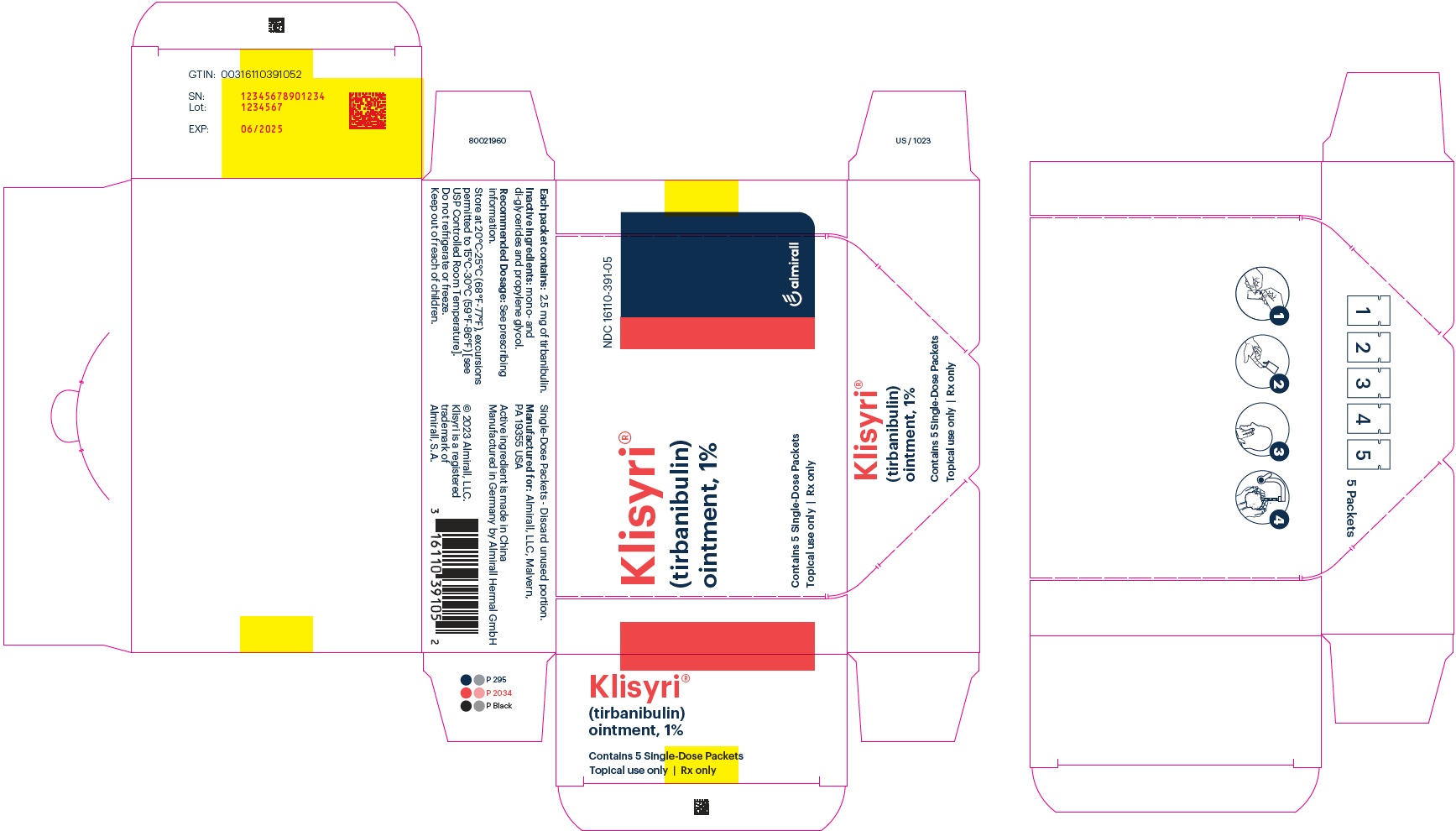
Klisyri
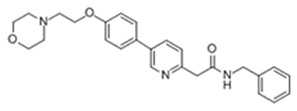
What is Klisyri (Tirbanibulin)?
Approved To Treat
Related Clinical Trials
Summary: The investigators seek to evaluate the effectiveness of fractional laser therapy and tirbanibulin ointment to treat squamous and basal cell carcinomas. This will be executed by using both thulium and erbium lasers on previously biopsy-confirmed SCCs and BCCs and applying bulk heating methods. Then, depending on the level of invasiveness, subjects may be instructed to apply the ointment over the co...
Summary: The aim of this study is to observe the influence of tirbanibulin on proliferation patterns of actinic keratoses (efficacy on proliferation score according to Schmitz et al.). For this purpose, tirbanibulin is applied in-label, proliferation is measured by LC-OCT at different time points and dermatohistopathology is performed (optionally) at the end. Local skin reactions to the product will also b...
Summary: Warts are common, benign skin lesions caused by the human papillomavirus (HPV). Treatment is challenging, particularly in the pediatric population, where standard modalities such as cryotherapy and intralesional immunotherapy are poorly tolerated. Existing topical treatments, such as imiquimod and 5-fluorouracil, have low efficacy and require prolonged use. Case reports suggest tirbanibulin ointme...
Related Latest Advances
Brand Information
- Wash hands well after applying KLISYRI to avoid transfer of the drug into the eyes and to the periocular area after application.
- Avoid washing and touching the treated area for 8 hours after treatment. Following this time, patients may wash the area with a mild soap and water
- Avoid inadvertent transfer of KLISYRI to other areas, or to another person.