Generic Name
Asenapine
Brand Names
Secuado, Saphris
FDA approval date: September 30, 2013
Classification: Atypical Antipsychotic
Form: Tablet, Film
What is Secuado (Asenapine)?
Asenapine sublingual Tablets are indicated for: Schizophrenia in adults [see Clinical Studies ( 1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
SECUADO (ASENAPINE)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SECUADO is not approved for the treatment of patients with dementia-related psychosis [
1INDICATIONS AND USAGE
SECUADO is indicated for the treatment of adults with schizophrenia [
2DOSAGE FORMS AND STRENGTHS
SECUADO (asenapine) transdermal system is a translucent rounded square product available in three dosage strengths:
- 3.8 mg asenapine / 24 hours
- 5.7 mg asenapine / 24 hours
- 7.6 mg asenapine / 24 hours
3CONTRAINDICATIONS
SECUADO is contraindicated in patients with:
- Severe hepatic impairment (Child-Pugh C)
- A history of hypersensitivity reactions to asenapine or any components of the transdermal system. Reactions with asenapine have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Use in Elderly Patients with Dementia-Related Psychosis
- Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
- Neuroleptic Malignant Syndrome
- Tardive Dyskinesia
- Metabolic Changes
- Hypersensitivity Reactions
- Orthostatic Hypotension, Syncope, and other Hemodynamic Effects
- Falls
- Leukopenia, Neutropenia, and Agranulocytosis
- QT Interval Prolongation
- Hyperprolactinemia
- Seizures
- Potential for Cognitive and Motor Impairment
- Body Temperature Regulation
- Dysphagia
- External Heat
- Application Site Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SECUADO was evaluated in a total of 315 adult patients diagnosed with schizophrenia who were exposed to SECUADO for up to 6 weeks in a placebo-controlled trial.
Adverse Reactions Leading to Discontinuation of Treatment
A total of 4.9% (10/204) patients treated with SECUADO 3.8 mg/24 hours, 7.8% (16/204) patients treated with SECUADO 7.6 mg/24 hours, and 6.8% (14/206) patients on placebo discontinued due to adverse reactions in the placebo-controlled trial. The adverse reaction that most commonly led to discontinuation among SECUADO-treated patients in this trial was akathisia, which led to discontinuation in no (0/204) patients treated with SECUADO 3.8 mg/24 hours, 1.5% (3/204) patients treated with SECUADO 7.6 mg/24 hours, and 0.5% (1/206) patients on placebo.
Commonly Observed Adverse Reactions
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported in adult patients with schizophrenia treated with SECUADO in the placebo-controlled trial were extrapyramidal disorder, application site reaction and weight gain.
Adverse Reactions Occurring at an Incidence of 2% or More in SECUADO-Treated Patients.
Adverse reactions associated with the use of SECUADO (incidence of ≥2%, rounded to the nearest percent, and SECUADO incidence greater than placebo) that occurred during the placebo-controlled trial are shown in
Dose-Related Adverse Reactions: In the placebo-controlled schizophrenia trial, the incidence of an extrapyramidal disorder and weight increased appear to be dose-related (see Table 5).
Dystonia:
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups
Extrapyramidal Symptoms:
In the short-term, placebo-controlled schizophrenia adult trial, data were objectively collected on the Simpson Angus Rating Scale for extrapyramidal symptoms (EPS), the Barnes Akathisia Scale (for akathisia) and the Assessments of Involuntary Movement Scales (for dyskinesias). The mean change from baseline for the SECUADO 3.8 mg/24 hours or 7.6 mg/24 hours treated group was similar to placebo in each of the rating scale scores.
In the short-term, placebo-controlled schizophrenia adult trial, the incidence of reported extrapyramidal disorder events, excluding events related to akathisia, was 7.8% for patients treated with SECUADO 3.8 mg/24 hours, 12.8% for patients treated with SECUADO 7.6 mg/24 hours and 2.4% for placebo-treated patients; and the incidence of akathisia-related events was 3.9% for patients treated with SECUADO 3.8 mg/24 hours, 4.4% for patients treated with SECUADO 7.6 mg/24 hours and 2.4% for placebo-treated patients.
Laboratory Test Abnormalities:
Transaminases: Transient elevations in serum transaminases (primarily ALT) were more common in SECUADO-treated patients. The mean increase in ALT levels for SECUADO-treated patients was 6.0 units/L and 3.8 units/L for the SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours treated groups, respectively, compared to a decrease of 1.1 units/L for placebo-treated patients. The proportion of patients with ALT elevations ≥3 times upper limit of normal (ULN) (at any time) was 1.6% and 3.1% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, and 0% for placebo-treated patients.
In a 52-week, double-blind, comparator-controlled trial that included primarily adult patients with schizophrenia, the mean increase from baseline of ALT was 1.7 units/L for sublingual asenapine.
Prolactin: The proportion of patients with prolactin elevations ≥4 times ULN (at Endpoint) were 0.0% and 1.3% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, as compared to 2.4% for placebo-treated patients in the short-term placebo-controlled trial.
In a long-term (52-week), double-blind, comparator-controlled adult trial that included primarily patients with schizophrenia, the mean decrease in prolactin from baseline for sublingual asenapine-treated patients was 26.9 ng/mL.
Creatine Kinase (CK): The proportion of adult patients with CK elevations ≥3 times ULN at any time were 1.6% and 2.1% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, as compared to 1.5% for placebo-treated patients in the short-term, placebo-controlled trial. The clinical relevance of this finding is unknown.
Other Adverse Reactions Observed During the Premarketing Evaluation of SECUADO
Other adverse reactions (<2% frequency) within the 6-week placebo-controlled trial in patients with schizophrenia are listed below. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions that appear elsewhere in the SECUADO label are not included.
Gastrointestinal disorders: vomiting, dry mouth
General disorders and administration site conditions: asthenia
Musculoskeletal and connective tissue disorders: myalgia
Other Adverse Reactions Reported in Clinical Trials with Sublingual Asenapine
Following is a list of MedDRA terms that reflect adverse reactions reported by patients treated with sublingual asenapine at multiple doses of ≥5 mg twice daily during any phase of a trial within the database of adult patients. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions already listed for adult patients in other parts of
Blood and lymphatic disorders: infrequent: anemia; rare: thrombocytopenia
Cardiac disorders: infrequent: temporary bundle branch block
Eye disorders: infrequent: accommodation disorder
Gastrointestinal disorders: infrequent: swollen tongue
General disorders: rare: idiosyncratic drug reaction
Investigations: infrequent: hyponatremia
Nervous system disorders: infrequent: dysarthria
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of sublingual asenapine and are possible with SECUADO treatment. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure.
- Choking has been reported by patients, some of whom may have also experienced oropharyngeal muscular dysfunction.
5OVERDOSAGE
Human Experience: In the placebo-controlled trial in adults for SECUADO, there were no reports of accidental or intentional acute overdosage of SECUADO.
In adult clinical studies involving more than 3350 patients and/or healthy subjects for sublingual asenapine, accidental or intentional acute overdosage of sublingual asenapine was identified in 3 patients. Among these few reported cases of overdose, the highest estimated ingestion of sublingual asenapine was 400 mg. Reported adverse reactions at the highest dosage included agitation and confusion.
Management of Overdosage: There is no specific antidote to SECUADO. The possibility of multiple drug involvement should be considered. An electrocardiogram should be obtained and management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Consult a Certified Poison Control Center at 1 800-222-1222 for up to date information on the management of overdosage.
Hypotension and circulatory collapse should be treated with appropriate measures, such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of SECUADO-induced alpha blockade). In case of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision and monitoring should continue until the patient recovers.
6DESCRIPTION
SECUADO transdermal system contains asenapine, an atypical antipsychotic.
Asenapine belongs to the class dibenzo-oxepino pyrroles. The chemical name is
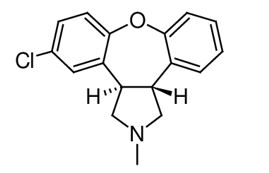
SECUADO is for transdermal administration and is provided in three strengths: 3.8 mg, 5.7 mg or 7.6 mg asenapine every 24 hours (
7CLINICAL STUDIES
The efficacy of SECUADO in the treatment of adult patients with schizophrenia was established, in part, on the basis of efficacy data from trials with the sublingual formulation of asenapine. In addition, the efficacy of SECUADO was evaluated in a 6-week, fixed-dose, randomized, double-blind, and placebo-controlled trial (Study 1; NCT 02876900) of adult patients who met DSM-IV criteria for schizophrenia.
In Study 1, the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impressions-Severity (CGI-S) rating scales were used as the primary and key secondary efficacy measures, respectively, for assessing psychiatric signs and symptoms in each trial:
- PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210.
- CGI-S is a validated clinician-rated scale that measures the patient’s current illness state and overall clinical state on a 1 (normal, not at all ill) to 7-point (extremely ill) scale, based on the rater’s total clinical experience with this population.
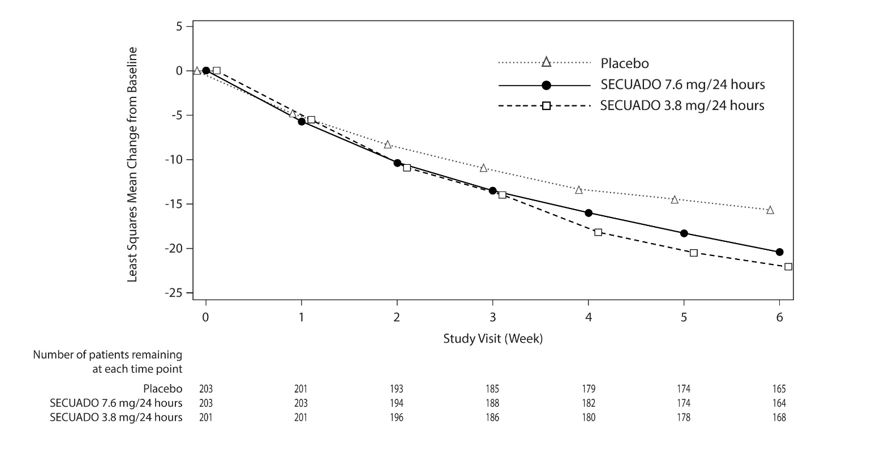
The primary endpoint was change from baseline in PANSS total score to Week 6. The change from baseline for SECUADO was compared to that for placebo. The results of the trial are shown in
In the 6-week trial (n=607) comparing two fixed doses of SECUADO (3.8 mg/24 hours and 7.6 mg/24 hours) to placebo, both doses were statistically superior to placebo for both PANSS total score and CGI-S.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex or race.
Figure 4: Change from Baseline in PANSS Total Score Over Time (Weeks) in Patients with Schizophrenia (Study 1)
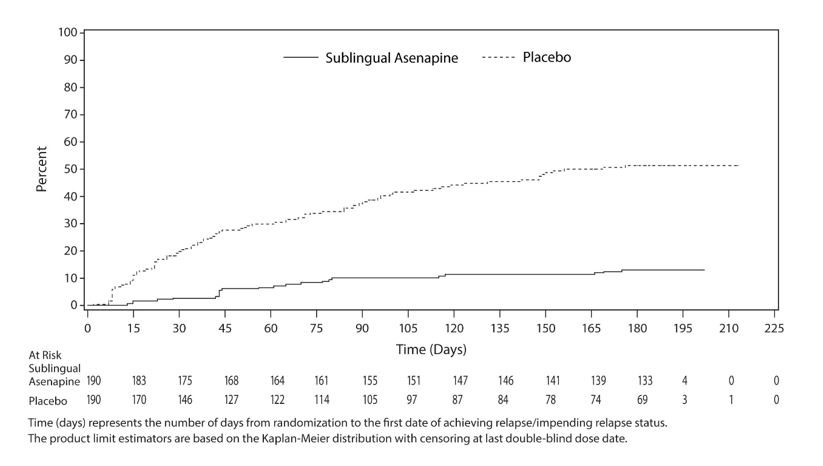
Maintenance of Efficacy with Sublingual Asenapine
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose with sublingual asenapine (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open label treatment with sublingual asenapine for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Sublingual asenapine was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a Clinical Global Impression–Severity of Illness (CGI-S) score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on "hostility" or "uncooperativeness" items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any two of the following items: "unusual thought content," "conceptual disorganization," or "hallucinatory behavior" items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons.The Kaplan-Meier curves of the time to relapse or impending relapse during the randomized, double-blind, placebo-controlled withdrawal phase of this trial for asenapine and placebo are shown in
Figure 5: Kaplan-Meier Estimation of Percent Relapse for Sublingual Asenapine and Placebo
Adhesion
Based on a clinical study in 40 subjects, each wearing one SECUADO 3.8 mg/24 hours, 40 transdermal systems (100%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every 4 hours) throughout the 24-hour wear period. Based on a clinical study in 39 subjects, each wearing one SECUADO 7.6 mg/24 hours, 36 transdermal systems (92%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every 4 hours) throughout the 24-hour wear period. One SECUADO 7.6 mg/24 hours transdermal system worn on the hip fully detached.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Hypersensitivity Reactions
Counsel patients on the signs and symptoms of a serious allergic reaction (e.g., difficulty breathing, itching, swelling of the face, tongue or throat, feeling lightheaded etc.) and to seek immediate emergency assistance if they develop any of these signs and symptoms [
Neuroleptic Malignant Syndrome
Counsel patients about a potentially fatal adverse reaction referred to as NMS that has been reported in association with administration of antipsychotic drugs. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS including hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their health care provider if these abnormal movements occur [
Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain)
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia (high blood sugar) and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight [
Orthostatic Hypotension
Counsel patients about the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing) especially early in treatment, and at times of re-initiating treatment or increases in dose [
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia they should have their CBC monitored while taking SECUADO [
Hyperprolactinemia
Counsel patients on the signs and symptoms of hyperprolactinemia and to contact their health care provider if these abnormalities occur [
Interference with Cognitive and Motor Performance
Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that SECUADO therapy does not affect them adversely [
Heat Exposure and Dehydration
Counsel patients regarding appropriate care in avoiding overheating and dehydration [
External Heat
Inform patients to avoid exposing SECUADO to external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., [
Application Site Reactions
Inform patients that application site reactions, including erythema, pruritus, papules, discomfort, pain, edema or irritation, have been reported with use of SECUADO. Inform patients that increased skin irritation may occur if applied for a longer period than instructed or if the same application site is used repeatedly. Instruct patients to select a different application site each day to minimize skin reactions. Patients should monitor for these reactions while wearing or immediately after removal of SECUADO [
Concomitant Medications
Advise patients to inform their health care provider if they are taking, or plan to take, any prescription or over-the-counter medications since there is a potential for interactions [
Pregnancy
Advise patients that SECUADO may cause fetal harm as well as extrapyramidal and/or withdrawal symptoms in a neonate. Advise patients to notify their healthcare provider with a known or suspected pregnancy [
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to SECUADO during pregnancy
Manufactured by: Hisamitsu Pharmaceutical Co. Inc., 408, Tashirodaikan-machi, Tosu, Saga, Japan
Distributed by: Noven Therapeutics, LLC, Miami, Florida USA.
For more information, call 1-800-455-8070 or visit www.secuado.com
SECUADO is a registered trademark of Hisamitsu Pharmaceutical Co., Inc.
©2019-2025 Hisamitsu Pharmaceutical Co., Inc. All rights reserved.
5O134A-0
9PRINCIPAL DISPLAY PANEL - NDC: 68968-0172-3 - Carton Label - 3.8 mg/24 hours
NDC 68968-0172-3
PUSH IN
PULL UP
Secuado
(asenapien)
transdermal system
3.8 mg/24 hours
For Transdermal Use Only
30 Transdermal Systems
Rx only
Each 20cm
Inactive ingredients: Alicylic saturated hydrocarbon resin, butylated hydroxytoluene, isopropyl palmitate, maleate salts (monosodium maleate and disodium maleate), mineral oil, polyester film backing, polyisobutylene, silicone-treated polyester release liner, sodium acetate anhydrous, and styrene-isoprene-styrene block copolymer.
Dosage and Administration: See package insert.
Important: Read Instructions for Use for application and proper use. Do not wear more than one Secuado transdermal system at a time. Apply immediately upon removal from pouch. To dispose, fold used transdermal system in half (sticky sides together) and place in trash right away.
Keep out of the reach of children.
Keep out of the reach of children.
Do not store unpouched. Store at room temperature at 20°C to 25°C (68°F to 77°F).
Find out more at www.secuado.com
1M418T-0
Manufactured by
Hisamitsu Pharmaceutical Co., Inc.
408, Tashirodaikan-machi, Tosu, Saga, Japan
Distributed by
Noven Therapeutics, LLC
Miami, FL 33186
10PRINCIPAL DISPLAY PANEL - NDC: 68968-0173-3 - Carton Label - 5.7 mg/24 hours
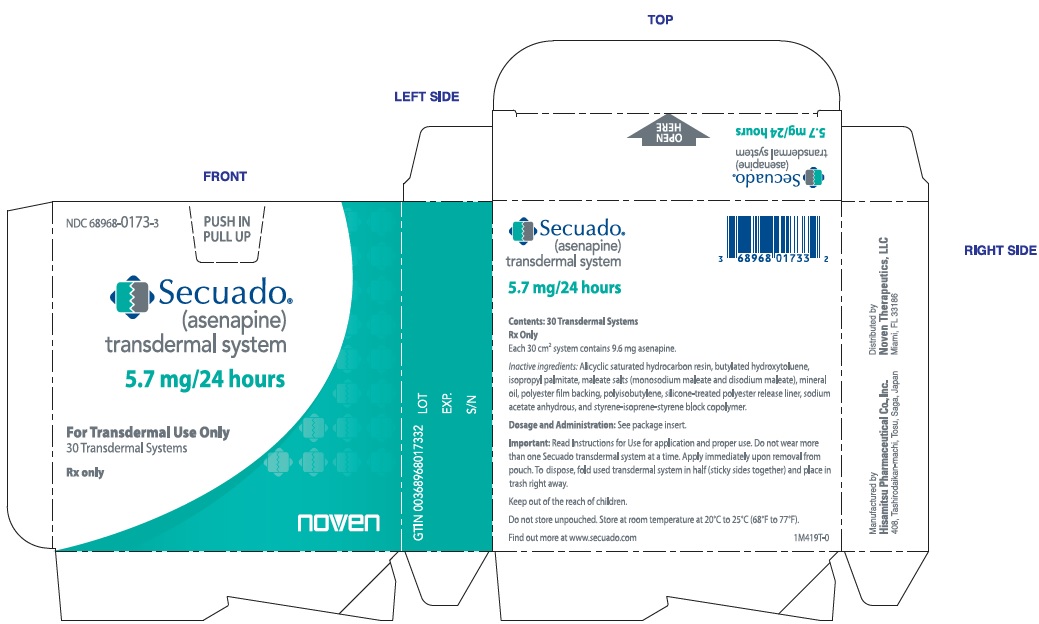
NDC 68968-0173-3
PUSH IN
PULL UP
Secuado
(asenapien)
transdermal system
5.7 mg/24 hours
For Transdermal Use Only
30 Transdermal Systems
Rx only
Each 30cm
Inactive ingredients: Alicylic saturated hydrocarbon resin, butylated hydroxytoluene, isopropyl palmitate, maleate salts (monosodium maleate and disodium maleate), mineral oil, polyester film backing, polyisobutylene, silicone-treated polyester release liner, sodium acetate anhydrous, and styrene-isoprene-styrene block copolymer.
Dosage and Administration: See package insert.
Important: Read Instructions for Use for application and proper use. Do not wear more than one Secuado transdermal system at a time. Apply immediately upon removal from pouch. To dispose, fold used transdermal system in half (sticky sides together) and place in trash right away.
Keep out of the reach of children.
Keep out of the reach of children.
Do not store unpouched. Store at room temperature at 20°C to 25°C (68°F to 77°F).
Find out more at www.secuado.com
1M419T-0
Manufactured by
Hisamitsu Pharmaceutical Co., Inc.
408, Tashirodaikan-machi, Tosu, Saga, Japan
Distributed by
Noven Therapeutics, LLC
Miami, FL 33186
11PRINCIPAL DISPLAY PANEL - NDC: 68968-0174-3 - Carton Label - 7.6 mg/24 hours

NDC 68968-0174-3
PUSH IN
PULL UP
Secuado
(asenapien)
transdermal system
7.6 mg/24 hours
For Transdermal Use Only
30 Transdermal Systems
Rx only
Each 40cm
Inactive ingredients: Alicylic saturated hydrocarbon resin, butylated hydroxytoluene, isopropyl palmitate, maleate salts (monosodium maleate and disodium maleate), mineral oil, polyester film backing, polyisobutylene, silicone-treated polyester release liner, sodium acetate anhydrous, and styrene-isoprene-styrene block copolymer.
Dosage and Administration: See package insert.
Important: Read Instructions for Use for application and proper use. Do not wear more than one Secuado transdermal system at a time. Apply immediately upon removal from pouch. To dispose, fold used transdermal system in half (sticky sides together) and place in trash right away.
Keep out of the reach of children.
Do not store unpouched. Store at room temperature at 20°C to 25°C (68°F to 77°F).
Find out more at www.secuado.com
1M420T-0
Manufactured by
Hisamitsu Pharmaceutical Co., Inc.
408, Tashirodaikan-machi, Tosu, Saga, Japan
Distributed by
Noven Therapeutics, LLC
Miami, FL 33186