Cholestyramine
What is Choleystyramine (Cholestyramine)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of each study is to independently measure the annualized relapse rate (ARR) with administration of frexalimab compared to a daily oral dose of teriflunomide in male and female participants with relapsing forms of multiple sclerosis (aged 18 to 55 years at the time of enrollment). People diagnosed with relapsing forms of multiple sclerosis are eligible for enrollment as long as they mee...
Summary: This phase I trial tests the safety and side effects of leflunomide in combination with steroids in treating patients with acute graft versus host disease who have undergone done stem cell transplant for blood cancers (hematologic malignancies). Sometimes the transplanted cells from a donor can attack the body's normal cells (called graft-versus-host disease). Leflunomide and steroids are immunosu...
Summary: This phase Ib trial tests the safety, side effects, and best dose of leflunomide in combination with gemcitabine in treating patients with pancreatic cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced) and cannot be removed by surgery (unresectable). Improving the effectiveness of gemcitabine without increasing side effects...
Related Latest Advances
Brand Information
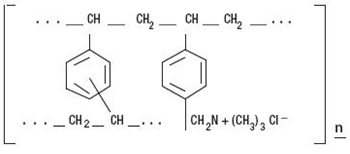
Inactive Ingredients: mannitol, fructose, sorbitol, aspartame, citric acid, lake pigment 6010 D&C yellow #10 aluminum lake and FD&C yellow #6/sunset yellow FCF AI 15% - 18%, orange flavour, propylene glycol alginate, xanthan gum, pectin, silicon dioxide.
In a large, placebo-controlled, multi-clinic study, LRC-CPPT1 , hypercholesterolemic subjects treated with cholestyramine resin had mean reductions in total and low-density lipoprotein cholesterol (LDL-C) which exceeded those for diet and placebo treatment by 7.2% and 10.4%, respectively. Over the seven year study period the cholestyramine resin group experienced a 19% reduction (relative to the incidence in the placebo group) in the combined rate of coronary heart disease death plus non-fatal myocardial infarction (cumulative incidences of 7% cholestyramine resin and 8.6% placebo). The subjects included in the study were men aged 35 to 59 with serum cholesterol levels above 265 mg/dL and no previous history of heart disease. It is not clear to what extent these findings can be extrapolated to females and other segments of the hypercholesterolemic population. (See also PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility.)
Preliminary evidence suggests that the lipid-lowering effects of cholestyramine on total and LDL-cholesterol are enhanced when combined with a HMG-CoA reductase inhibitor, e.g., pravastatin, lovastatin, simvastatin and fluvastatin. Additive effects on LDL-cholesterol are also seen with combined nicotinic acid/cholestyramine therapy. See the PRECAUTIONS, Drug Interactions for recommendations on administering concomitant therapy.
The color of Cholestyramine for Oral Suspension USP Light powder may vary somewhat from batch to batch but this variation does not affect the performance of the product. Place the contents of one single-dose pouch or one level scoopful of Cholestyramine for Oral Suspension USP Light powder in a glass or cup. Add at least 2 to 6 ounces of water or other noncarbonated beverage of your choice. Stir to a uniform consistency and drink.
Cholestyramine for Oral Suspension USP Light powder may also be mixed with highly fluid soups or pulpy fruits with a high moisture content such as applesauce or crushed pineapple.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
1. The Lipid Research Clinics Coronary Primary Prevention Trial Results: (I) Reduction in Incidence of Coronary Heart Disease; (II) The Relationship of Reduction in Incidence of Coronary Heart Disease to Cholesterol Lowering. JAMA. 1984; 251:351-374.
Alkem Laboratories Ltd.,
INDIA.
339 Jefferson Road,
Parsippany, NJ 07054
Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per scoopful.
Orange Flavor

Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per pouch.
Orange Flavor
NDC 67877-422-57
Cholestyramine for Oral Suspension USP Light Powder
4 grams cholestyramine resin USP, per pouch.
Orange Flavor