Brand Name
Recorlev
Generic Name
Levoketoconazole
View Brand Information FDA approval date: December 30, 2021
Classification: Cortisol Synthesis Inhibitor
Form: Tablet
What is Recorlev (Levoketoconazole)?
RECORLEV is indicated for the treatment of endogenous hypercortisolemia in adult patients with Cushing’s syndrome for whom surgery is not an option or has not been curative. Limitations of Use RECORLEV is not approved for the treatment of fungal infections. The safety and effectiveness of RECORLEV for the treatment of fungal infections have not been established. RECORLEV is a cortisol synthesis inhibitor indicated for the treatment of endogenous hypercortisolemia in adult patients with Cushing’s syndrome for whom surgery is not an option or has not been curative Limitations of Use RECORLEV is not approved for the treatment of fungal infections
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Recorlev (Levoketoconazole)
WARNING: HEPATOTOXICITY AND QT PROLONGATION
Hepatotoxicity
• Cases of hepatotoxicity with a fatal outcome or requiring liver transplantation have been reported with use of oral ketoconazole. Some patients had no obvious risk factors for liver disease. Serious hepatotoxicity has been reported in patients receiving RECORLEV
• RECORLEV is contraindicated in patients with cirrhosis, acute liver disease or poorly controlled chronic liver disease, recurrent symptomatic cholelithiasis, a prior history of drug induced liver injury due to ketoconazole or any azole antifungal therapy that required discontinuation of treatment, or extensive metastatic liver disease
• Evaluate liver enzymes prior to and during treatment. Interrupt RECORLEV treatment immediately if signs of hepatotoxicity occur
QT Prolongation
• RECORLEV is associated with dose-related QT interval prolongation. QT interval prolongation may lead to life-threatening ventricular dysrhythmias such as torsades de pointes
• RECORLEV is associated with dose-related QT interval prolongation. QT interval prolongation may lead to life-threatening ventricular dysrhythmias such as torsades de pointes
• Coadministration of RECORLEV with other drugs that prolong the QT interval associated with ventricular arrhythmias, including torsades de pointes, and use in patients with a prolonged QTcF interval of greater than 470 msec at baseline, history of torsades de pointes, ventricular tachycardia, ventricular fibrillation, or long QT syndrome (including first-degree family history) are contraindicated
• Perform an ECG and correct hypokalemia and hypomagnesemia prior to and during treatment. Temporarily discontinue RECORLEV if QTcF interval exceeds 500 msec
1INDICATIONS AND USAGE
RECORLEV is indicated for the treatment of endogenous hypercortisolemia in adult patients with Cushing’s syndrome for whom surgery is not an option or has not been curative.
Limitations of Use
RECORLEV is not approved for the treatment of fungal infections. The safety and effectiveness of RECORLEV for the treatment of fungal infections have not been established.
2DOSAGE FORMS AND STRENGTHS
Tablets: 150 mg, round, pink, film-coated, and imprinted in black ink with “LEV” above “150” on one side; the other side is plain.
3CONTRAINDICATIONS
RECORLEV is contraindicated in patients:
- With cirrhosis, acute liver disease or poorly controlled chronic liver disease, baseline AST or ALT greater than 3 times the upper limit of normal, recurrent symptomatic cholelithiasis, a prior history of drug induced liver injury due to ketoconazole or any azole antifungal therapy that required discontinuation of treatment, or extensive metastatic liver disease
- Taking drugs that cause QT prolongation associated with ventricular arrhythmias, including torsades de pointes
- With a prolonged QTcF interval of greater than 470 msec at baseline, history of torsades de pointes, ventricular tachycardia, ventricular fibrillation, or long QT syndrome (including first-degree family history)
- With known hypersensitivity to levoketoconazole, ketoconazole or any excipient in RECORLEV
- Taking certain drugs that are sensitive substrates of CYP3A4 or CYP3A4 and P-gP
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hepatotoxicity
- QT Prolongation
- Hypocortisolism
- Hypersensitivity Reactions
- Risks related to Decreased Testosterone
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RECORLEV was evaluated in a multicenter, randomized-withdrawal study (Study 1) and in a multicenter, single-arm, open-label study (Study 2). During the two studies, 166 patients were exposed to RECORLEV, of which 104 patients were exposed for more than 6 months and 51 patients were exposed for at least 1 year. In both studies, most patients took RECORLEV twice daily in total daily dosages ranging from 300 mg to 1200 mg
Adverse reactions, excluding hepatic injury, reported in ≥10% of patients treated with RECORLEV in Study 1 are presented in
Other notable adverse reactions which occurred with a frequency less than 10% during Study 1 were: alopecia (6%), gastrointestinal infection (6%), urinary tract infection (6%), hypogonadism (2%), and hypersensitivity (1%).
Adverse reactions, excluding hepatic injury, reported in ≥10% of patients treated with RECORLEV in Study 2 are presented in
Other notable adverse reactions which occurred with a frequency less than 10% during Study 2 were: gastrointestinal infections (5%), decreased libido (5%), hypogonadism (4%), adrenal insufficiency (3%), and gynecomastia (3%).
Description of Selected Adverse Reactions
Hepatic Injury and Elevated Liver Function Tests
Liver-related adverse reactions reported in patients treated with RECORLEV in Studies 1 and 2 are presented in
QTc Interval Prolongation
In Study 1 and 2, there were 4 (2.4%) patients who experienced QTcF>500 msec, and 23 (14.7%) patients who experienced change-from-baseline QTcF >60 msec, respectively
Hypocortisolism
Hypocortisolism was reported in 11 (7%) of 166 patients across Studies 1 and 2, with events starting on median study day 96 (range 26-166). The majority of cases were managed by reducing the dosage or temporarily interrupting treatment with RECORLEV.
4.2Postmarketing Experience
The following adverse reactions have been identified from published reports or postmarketing experience with ketoconazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to ketoconazole exposure.
Blood and Lymphatic System Disorders: thrombocytopenia
Endocrine Disorders:adrenocortical insufficiency
Hepatobiliary Disorders:serious hepatotoxicity including hepatitis cholestatic, biopsy-confirmed hepatic necrosis, cirrhosis, hepatic failure including cases resulting in transplantation or death
Immune System Disorders:allergic conditions including anaphylactic shock, anaphylactic reaction, angioneurotic edema
Nervous System Disorders:reversible intracranial pressure increased (e.g., papilledema, fontanelle bulging in infants)
Reproductive System and Breast Disorders:erectile dysfunction; with dosages higher than 200 or 400mg daily, azoospermia.
Skin and Subcutaneous Tissue Disorders:acute generalized exanthematous pustulosis, photosensitivity
5OVERDOSAGE
In the event of acute accidental overdose, treatment consists of supportive and symptomatic measures. Within the first hour after ingestion, activated charcoal may be administered.
6DESCRIPTION
RECORLEV (levoketoconazole) tablets contain levoketoconazole as the active ingredient. Levoketoconazole is the 2S,4R- enantiomer derived from racemic ketoconazole and is a cortisol synthesis inhibitor.
The chemical name of levoketoconazole is 2S,4R cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl] methoxyl]phenyl] piperazine.
The molecular formula of levoketoconazole is C
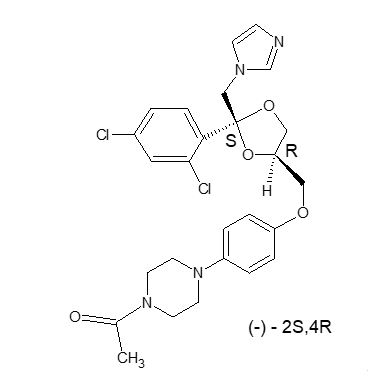
Levoketoconazole is a white or almost white crystalline powder. It is very slightly soluble in water but soluble in aqueous solutions below pH 2.
RECORLEV tablets for oral administration contain 150 mg of levoketoconazole and the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, modified corn starch, and silicified microcrystalline cellulose. The non-functional pink film-coating contains iron oxide red, macrogol/polyethylene glycol 3350, polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide. The tablets are printed with a black imprinting ink that contains ammonium hydroxide 28%, ferrosoferric oxide, isopropyl alcohol, propylene glycol, and shellac glaze 45% (20% esterified) in ethanol.
7CLINICAL STUDIES
The effectiveness of RECORLEV in patients with Cushing's syndrome was evaluated in two studies, Study 1 and Study 2.
Study 1
Study 1 consisted of an open-label dose titration and maintenance phase of up to 19 weeks duration, followed by an 8-week double-blind, placebo-controlled, randomized withdrawal phase (
Study 1 enrolled 84 Cushing’s syndrome patients with persistent or recurrent disease despite surgery, previously medically treated patients, and previously untreated patients. The etiology of Cushing's syndrome was Cushing's disease for 70 (83%) patients, adrenal Cushing’s syndrome in 8 (10%) patients, ectopic ACTH secretion for 2 (2%) patients, and unknown for 4 (5%) patients. Patients with pituitary or adrenal carcinoma were excluded. Twelve (14%) patients who had previously received RECORLEV in Study 2 were also enrolled in Study 1. The mean age at baseline was 45 years; 76% of patients were female. Overall, the mean time since diagnosis was 63 months before treatment with the first dose in this study. Persistence or recurrence of Cushing’s syndrome was evidenced by the mean of three 24-hour UFC levels greater than or equal to 1.5 × upper limit of normal (normal range: 11 to 138 nmol/day or 4 to 50 µg/day). For the 79 patients who underwent dose titration, the mean mUFC (SD) at study baseline was 785 nmol/day (932), which corresponds to approximately 6 × ULN. The median mUFC at baseline was 479 nmol/day, which corresponds to approximately 3.5 × ULN. Seventy-two (72) patients were naïve to treatment with RECORLEV, seven (7) patients were treated with RECORLEV in Study 2 but were not on therapeutic dose (dose at which mUFC level was ≤ ULN, or maximum allowed dose [600 mg twice daily] had been reached, or a clinically meaningful partial response based on clinical judgement, and the maximum tolerated dose had been reached) prior to the enrollment in Study 1. Five (5) of 84 patients continued treatment with therapeutic dose of RECORLEV prior to the enrollment in Study 1; these patients were enrolled directly in the randomized withdrawal phase.
Dose Titration and Maintenance Phase (14-19 weeks)
Seventy-nine (79) patients entered the dose titration and maintenance phase. Patients naïve to treatment with RECORLEV were started on 150 mg of RECORLEV orally twice daily. Patients who previously participated in Study 2 could start on a dose higher than 150 mg twice daily. The dose could be titrated in 150-mg increments at 2-week intervals to a maximum of 600 mg twice daily to achieve mUFC within the normal range. The dose was increased if mUFC was above ULN and was reduced based on individual tolerability. Patients who achieved a stable therapeutic dose for at least 4 weeks and achieved a normal mUFC at the end of the dose titration and maintenance phase were eligible for the randomized withdrawal phase.
Randomized Withdrawal Phase (approximately 8 weeks)
Forty-four (44) patients entered the randomized withdrawal phase: 39 patients from dose titration and maintenance phase and 5 patients directly from Study 2. Patients were randomized in a 1:1 ratio to either continue RECORLEV or receive matched placebo for approximately 2 months or until early rescue was necessary (i.e., for mUFC >1.5 × ULN).
Efficacy Assessment and Results
The key secondary efficacy endpoint was the proportion of patients with mUFC normalization, defined as a patient with mUFC at or below the ULN at the end of randomized withdrawal phase without meeting a requirement for early rescue during the randomized withdrawal phase.
Out of the 79 patients who entered the dose titration and maintenance phase, 37 (47%) patients who met the requirement to be on a stable therapeutic dose for at least 4 weeks and established normal mUFC at the end of the dose titration and maintenance phase, and 2 patients who did not meet the requirement due to abnormal mUFC, continued to the randomized-withdrawal phase. Out of 5 patients from Study 2 who were enrolled directly in the randomized withdrawal phase, 2 patients had normal mUFC.
Among the 39 patients who had normal mUFC at the randomized withdrawal phase baseline, 21 were randomized to the RECORLEV group and 18 to the placebo group. The number and percent of patients who had normal mUFC at the end of the randomized withdrawal phase was 11/21 (52.4%) in RECORLEV group and 1/18 (5.6%) in placebo group, and the treatment difference (CI) was 46.8% (16.5%, 70.2%). Out of 11 patients with normal mUFC at the end of the randomized-withdrawal phase, 7 patients in the RECORLEV group had normal mUFC throughout the randomized-withdrawal phase. Figure 1 depicts the mUFC during the randomized-withdrawal phase of Study 1. The line for the placebo group should be interpreted with caution as majority of placebo patients were rescued early due to high mUFC levels and were not included in the analysis.
Figure 1: Line Plot of the Mean Urinary Free Cortisol During the Randomized Withdrawal Phase of Study 1 - Observed Mean (± SE)
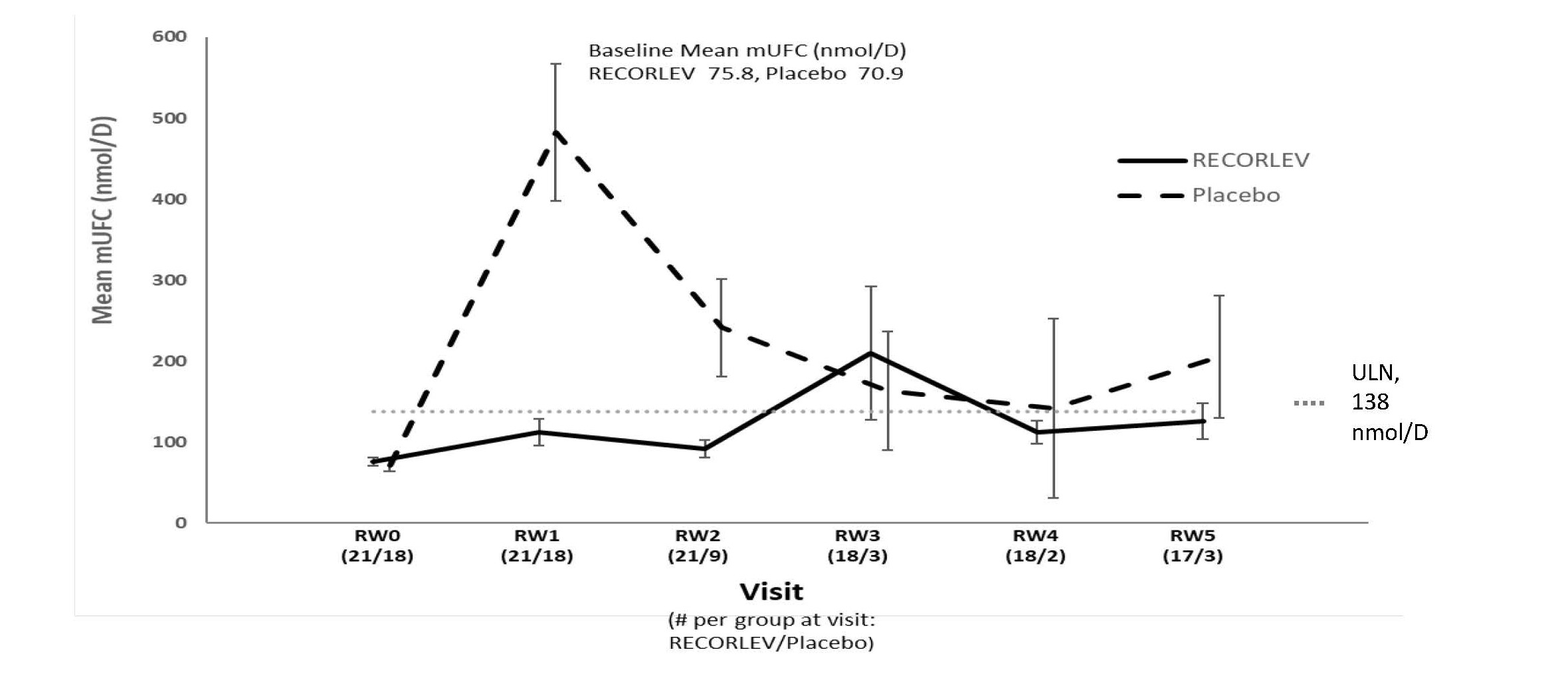
mUFC = Mean Urinary Free Cortisol; RW = Randomized Withdrawal; SE = Standard Error; ULN = Upper Limit of Normal
Only patients who remained in study with non-missing mUFC are included in the analysis
Study 2
Supportive evidence of efficacy was obtained from Study 2 which was a multicenter, single-arm, open-label study that consisted of three study phases (dose titration, maintenance, and extended evaluation) for a total estimated treatment duration of up to 73 weeks (
Study 2 enrolled 94 Cushing’s syndrome patients naïve to the treatment with RECORLEV with persistent or recurrent disease despite surgery, previously medically treated patients, and previously untreated patients. The etiology of Cushing's syndrome was benign pituitary adenoma for 80 (85%) patients, adrenal Cushing’s syndrome in 8 (9%) patients, ectopic ACTH secretion for 1 (1%) patient, and unknown source for 5 (5%) patients. Patients with pituitary or adrenal carcinoma were excluded. The mean age at enrollment was 44 years; 82% of patients were female. Overall, the mean time since diagnosis was 68 months before treatment with the first dose in this study. Persistence or recurrence of Cushing’s syndrome was evidenced by the mean of four 24-hour UFC (mUFC) levels greater than or equal to 1.5 times upper limit of normal (ULN); normal range: 11 to 138 nmol/day or 4 to 50 µg/day). The mean (SD) of the mean urinary free cortisol (mUFC) at baseline was 243 µg/day (269), which corresponds to approximately 5 x ULN. The median mUFC at baseline was 148 µg/day (range 59-1510), which corresponds to approximately 3 x ULN.
Dose Titration Phase (2 to 21 weeks)
Ninety-four (94) patients received a starting dosage of 150 mg RECORLEV orally twice daily that was titrated approximately every 2 to 3 weeks if mUFC was above the ULN to a maximum of 600 mg twice daily. Patients who achieved a therapeutic dose were continued to the maintenance phase. Therapeutic dose was defined as a dose at which mUFC level was ≤ ULN, or maximum allowed dose (600 mg twice daily) had been reached, or a clinically meaningful partial response based on clinical judgement, and the maximum tolerated dose had been reached.
Maintenance Phase (6 months)
Seventy-seven (77) patients who achieved a therapeutic dose in the dose titration phase entered the maintenance phase and continued treatment with therapeutic dose of RECORLEV for 6 months. The dose of RECORLEV was allowed to be decreased for safety or tolerability reasons or increased for loss of efficacy. The primary efficacy endpoint was evaluated at the end of maintenance phase.
Extended Evaluation Phase (6 months)
Sixty (60) patients entered the extended evaluation phase in which treatment with RECORLEV continued for an additional 6 months.
Efficacy Assessment and Results
The primary efficacy endpoint of the study was the proportion of patients with normalization of mUFC at the end of the 6-month maintenance phase. Normalization of mUFC was defined as mUFC at or below the ULN based on central laboratory result without requiring a dose increase during the maintenance phase. At the end of the maintenance phase, 29 of 94 patients (30.9%, 95% exact confidence interval 21.7%, 41.2%) met the primary endpoint.
Out of the 94 patients who enrolled in Study 2, 63 (67%) patients had normal mUFC at the end of the titration phase, 29 (30.9%) patients had normal mUFC at the end of the maintenance phase without any dose increase during the maintenance phase, and 16 (17%) patients had normal mUFC at the end of the extended evaluation phase without dose increase during maintenance or extended evaluation phase. However, because 51% of patients discontinued treatment prematurely due to adverse reaction, lack of efficacy, or other reasons, these results should be interpreted with caution.
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
RECORLEV (levoketoconazole) tablets, 150 mg are round, biconvex tablets, with a pink-colored film coating, containing 150 mg of levoketoconazole, and imprinted with an identification code in black ink with the “LEV” printed above the “150” on one side. The other side is plain.
Bottles of 50 with child-resistant closure: NDC 72065-003-01
Storage
Store RECORLEV at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) (see USP controlled room temperature).
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Monitoring
Instruct patients on the importance of laboratory monitoring and adherence to their return-visit schedule
Liver Injury
Inform patients that RECORLEV may cause liver injury. Advise patients of the signs and symptoms of hepatotoxicity (e.g., right upper quadrant pain associated with nausea, unusual fatigue, signs of jaundice, unusual bruising or bleeding). Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity. Advise patients that liver tests will be measured before treatment and periodically thereafter. Advise patients to avoid excessive alcohol use while taking RECORLEV
QT Prolongation
Inform patients that RECORLEV may cause QT prolongation. Advise patients to contact their healthcare provider immediately for signs or symptoms of QT prolongation, which include severe lightheadedness (pre-syncope) or fainting (syncope). Advise patients that an ECG will be taken before treatment and periodically thereafter. Advise patients that potassium and magnesium disturbances may require correction to aid in preventing QT interval prolongation
Hypocortisolism
Inform patients that RECORLEV may cause hypocortisolism. Advise patients of the signs and symptoms of hypocortisolism. Advise patients to report signs and symptoms of hypocortisolism to their healthcare provider promptly. Advise patients that cortisol in the blood or urine will be measured before treatment and periodically thereafter
Hypersensitivity Reactions
Inform patients that RECORLEV may cause hypersensitive reactions. Advise patients to contact their healthcare provider if signs or symptoms of hypersensitivity reaction occur
Drug Interactions
Inform patients that RECORLEV may interact with many drugs. Advise patients to report to their healthcare provider the use of all prescription and nonprescription medications
Pregnancy
Advise pregnant patients and females of reproductive potential of the risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
Lactation
Advise patients not to breastfeed during treatment with RECORLEV and for one day after the final dose
Infertility
Advise patients of reproductive potential that RECORLEV may impair fertility
10MEDICATION GUIDE RECORLEV ®
MEDICATION GUIDE RECORLEV® (re kor ' lev) (levoketoconazole) tablets, for oral use
What is the most important information I should know about RECORLEV?
RECORLEV can cause serious side effects, including:
- Liver damage (hepatotoxicity).Hepatotoxicity can happen in people who take RECORLEV. Some people who are treated with ketoconazole, a medicine like the active ingredient in RECORLEV, had serious liver problems that required a liver transplant or led to death.
Call your healthcare provider right away if you have any of the following signs or symptoms:
- pain on the upper right side of your stomach area (abdomen) associated with nausea
- unusual fatigue
- yellowing of your skin or the whites of your eyes (jaundice)
- unusual bruising or bleeding
RECORLEV should not be used if you have any of the following conditions:
- cirrhosis
- active or poorly controlled liver disease
- frequent stones in your gallbladder (cholelithiasis)
- history of liver problems due to use of a drug
Your healthcare provider will do liver tests before and during treatment with RECORLEV.
- Heart rhythm problems (QT prolongation).RECORLEV can cause a heart problem called QT interval prolongation, or QT prolongation. QT prolongation can cause irregular heartbeats that can be life threatening.
Call your healthcare provider right away ifyou feel severe lightheadedness or if you faint during treatment with RECORLEV.
- Low blood electrolyte levels of potassium and magnesium can increase your chances of QT prolongation during treatment with RECORLEV.
Your healthcare provider will check your heart with a test called an electrocardiogram (ECG) and do blood tests to check your blood electrolyte levels before and during treatment with RECORLEV.
RECORLEV should not be taken with certain other medicines that cause QT prolongation. Talk to your healthcare provider about the medicines you are taking before you start taking RECORLEV.
- Low cortisol levels (adrenal insufficiency).Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones, such as cortisol. RECORLEV may cause adrenal function insufficiency by excessively lowering production of cortisol.
Call your healthcare provider right away ifyou have one or more of the following symptoms, as these may be symptoms of reduced adrenal function:
- nausea or vomiting
- dizziness
- unusual fatigue
- low blood pressure (hypotension)
- unexplained stomach pain (abdomen)
- abnormal electrolyte levels
- loss of appetite
- low blood sugar (hypoglycemia)
- body aches
Your healthcare provider will collect blood or urine samples to measure your cortisol.
What is RECORLEV?
- RECORLEV is a prescription medicine used to treat high cortisol (endogenous hypercortisolemia) levels in adult patients with Cushing’s syndrome (CS) who cannot have surgery or who have had surgery that did not cure their Cushing's syndrome.
- It is not known if RECORLEV is safe and effective for the treatment of fungal infections. RECORLEV is not to be used for treatment of fungal infections.
Do not take RECORLEV if you:
- have or have had liver problems.
- take certain other medicines that cause QT prolongation.
- have a history of certain heart problems which may include one the following conditions: torsades de pointes, ventricular tachycardia, ventricular fibrillation, or long QT syndrome.
- are allergic to levoketoconazole, ketoconazole, or any of the ingredients in RECORLEV. See the end of this Medication Guide for a complete list of ingredients in RECORLEV.
- take certain medicines that may affect how your liver works (for example, CYP3A4 inhibitors). If you are not sure if you take these medicines, please ask your healthcare provider.
Before taking RECORLEV, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had liver problems.
- have any heart problems, including a condition called long QT syndrome.
- have a history of low blood levels of potassium or magnesium.
- are pregnant or plan to become pregnant. RECORLEV may harm your unborn baby. Tell your healthcare provider right away if you become pregnant during treatment with RECORLEV or think you might be pregnant.
- are breastfeeding or plan to breastfeed. RECORLEV can pass into your breast milk. You and your healthcare provider should decide if you should take RECORLEV or breastfeed. You should not breastfeed during treatment with RECORLEV and for 1 day after final dose.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. RECORLEV and other medicines may affect each other causing side effects. RECORLEV may affect the way other medicines work, and other medicines may affect how RECORLEV works.
How should I take RECORLEV?
- Take RECORLEV exactly as your healthcare provider tells you. Your healthcare provider will tell you how much RECORLEV to take and when to take it.
- RECORLEV is taken by mouth 2 times a day, with or without food.
- Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with RECORLEV if needed.
- If you miss a dose of RECORLEV, take the next dose at your regular scheduled time.
- If you take too much RECORLEV, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking RECORLEV?
- Drinking alcohol to excess while taking RECORLEV may increase your chances of having serious side effects.
What are the possible side effects of RECORLEV?
RECORLEV may cause serious side effects, including:
- See
- Hypersensitivity reactions.Serious allergic reactions can happen in people who take RECORLEV. Call your healthcare provider right away, or visit an emergency center, if you get a rash, itching, hives, fever, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
- Risks related to decreased testosterone.RECORLEV may lower testosterone levels in males and females. Call your healthcare provider if you have any of these symptoms:
- Males: breast enlargement (gynecomastia) and erectile dysfunction (impotence)
- Females: low desire for sex (decreased libido) and mood changes.
The most common side effects of RECORLEV includenausea/vomiting, hypokalemia (low potassium), hemorrhage (easy bleeding)/contusion (easy bruising), high blood pressure, headache, liver injury, abnormal uterine bleeding, erythema (redness of the skin), fatigue, abdominal pain/dyspepsia (upset stomach), arthritis, upper respiratory infection, myalgia (muscle pain), abnormal heart rhythm, back pain, sleep disturbances, and peripheral edema (fluid retention).
RECORLEV may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of RECORLEV.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store RECORLEV?
- Store RECORLEV at room temperature between 68°F to 77°F (20°C to 25°C).
Keep RECORLEV and all medicines out of the reach of children.
General information about the safe and effective use of RECORLEV.
Medications are sometimes prescribed for purposes other than those listed in this Medication Guide. Do not use RECORLEV for a condition for which it was not prescribed. Do not give RECORLEV to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about RECORLEV that is written for health professionals.
What are the ingredients in RECORLEV?
Active ingredients:levoketoconazole.
Inactive ingredients:colloidal silicon dioxide, lactose monohydrate, magnesium stearate, modified corn starch, and silicified microcrystalline cellulose.
Distributed by: Xeris Pharmaceuticals, Inc., Chicago, IL 60607
For more information, go to www.Recorlev.com or call 1-877-937-4737.
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 06/2023
11Principal Display Panel-Bottle Label (50 Tablets)
NDC 72065-003-01
Recorlev®
(levoketoconazole)
Tablets 150 mg
Dispense the Medication Guide

