Brand Name
Cefepime
View Brand InformationFDA approval date: May 01, 2008
Classification: Cephalosporin Antibacterial
Form: Injection
What is Cefepime?
Cefepime Injection is a cephalosporin antibacterial indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms: pneumonia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Cefepime (Cefepime)
1DOSAGE FORMS AND STRENGTHS
Cefepime for Injection, USP is a sterile white to pale yellow powder of cefepime in single-dose vials for reconstitution and it is available in the following strengths:
• 0.5 gram per vial
• 1 gram per vial
• 2 grams per vial
2CONTRAINDICATIONS
Cefepime for Injection is contraindicated in patients who have shown immediate hypersensitivity reactions to cefepime or the cephalosporin class of antibacterial drugs, penicillins or other beta-lactam antibacterial drugs.
3ADVERSE REACTIONS
The following adverse reactions are discussed in the Warnings and Precautions section and below:
- Hypersensitivity Reactions [
- Neurotoxicity [
- Clostridioides difficile-Associated Diarrhea [see ]
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials using multiple doses of cefepime, 4137 patients were treated with the recommended dosages of cefepime (500 mg to 2 g intravenous every 12 hours). There were no deaths or permanent disabilities thought related to drug toxicity. Sixty-four (1.5%) patients discontinued medication due to adverse reactions. Thirty-three (51%) of these 64 patients who discontinued therapy did so because of rash. The percentage of cefepime-treated patients who discontinued study drug because of drug-related adverse reactions was similar at daily doses of 500 mg, 1 g, and 2 g every 12 hours (0.8%, 1.1%, and 2%, respectively). However, the incidence of discontinuation due to rash increased with the higher recommended doses.
The following adverse reactions (
At the higher dose of 2 g every 8 hours, the incidence of adverse reactions was higher among the 795 patients who received this dose of cefepime. They consisted of rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%), and headache (1%).
The following (
A similar safety profile was seen in clinical trials of pediatric patients
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Cefepime for Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In addition to the adverse reactions reported during the North American clinical trials with cefepime, the following adverse reactions have been reported during worldwide postmarketing experience. Encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), aphasia, myoclonus, seizures, and nonconvulsive status epilepticus have been reported. [
Anaphylaxis including anaphylactic shock, transient leukopenia, neutropenia, agranulocytosis and thrombocytopenia, have been reported.
3.3Cephalosporin-Class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with cefepime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibacterial drugs:
Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal dysfunction, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, hepatic dysfunction including cholestasis, and pancytopenia.
4OVERDOSAGE
Patients who receive an overdose should be carefully observed and given supportive treatment. In the presence of renal insufficiency, hemodialysis, not peritoneal dialysis, is recommended to aid in the removal of cefepime from the body. Symptoms of overdose include encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, neuromuscular excitability and nonconvulsive status epilepticus [
5DESCRIPTION
Cefepime for Injection, USP (cefepime hydrochloride, USP) is a semi-synthetic, cephalosporin antibacterial for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride,7
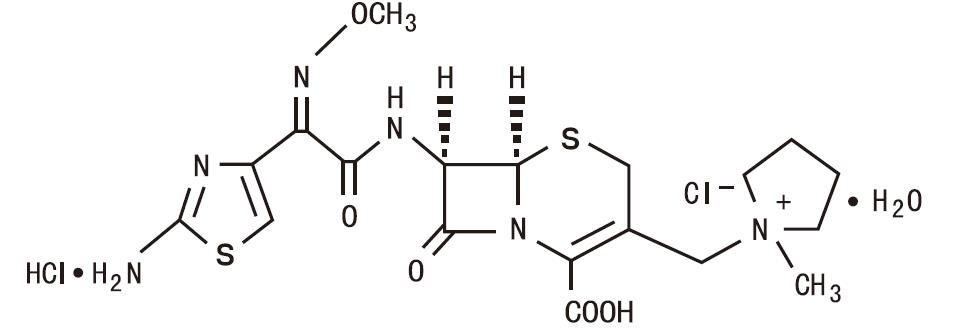
Cefepime hydrochloride is a white to pale yellow powder. Cefepime hydrochloride contains the equivalent of not less than 825 mcg and not more than 911 mcg of cefepime (C
Cefepime for Injection, USP is supplied for intramuscular or intravenous administration in strengths equivalent to 500 mg, 1 g, and 2 g of cefepime. Cefepime for Injection, USP is a sterile, dry mixture of cefepime hydrochloride and L-arginine. The L-arginine, at an approximate concentration of 707 mg/g of cefepime, is added to control the pH of the constituted solution at 4 to 6.
6REFERENCES
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31-41.
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Cefepime for injection, USP is supplied as follows: Cefepime for Injection, USP in the dry state, is a white to pale yellow powder. Constituted solution of Cefepime for Injection, USP can range in color from pale yellow to amber.
Storage and Handling
Cefepime for injection, USP in the dry state should be stored at 20 to 25°C (68 to 77°F) [see USP controlled room temperature.] and protected from light.
8PATIENT COUNSELING INFORMATION
•Counsel patients that antibacterial drugs including Cefepime for Injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefepime for Injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefepime for Injection or other antibacterial drugs in the future.
•Diarrhea is a common problem caused by antibacterial drugs, which usually ends when the antibacterial drug is discontinued. Inform patient that they may develop watery and bloody stools (with or without stomach cramps and fever) during treatment and as late as two or more months after having taken the last dose of the antibacterial drug. Inform patients that they should contact their physician as soon as possible if this occurs.
•Advise patients of neurological adverse events that could occur with Cefepime for Injection use. Instruct patients or their caregivers to inform their healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), aphasia (disturbance of speaking and understanding spoken and written language), myoclonus, seizures and nonconvulsive status epilepticus, for immediate treatment, dosage adjustment, or discontinuation of Cefepime for Injection.
Manufactured by:
Qilu Pharmaceutical Co., Ltd.
High Tech Zone
Jinan, 250101, China
Qilu Antibiotics Pharmaceutical Co., Ltd.
Jinan, 250105, China
Manufactured for:
Apotex Corp.
Weston, Florida, USA 33326
Code number: 34040006111E
Code number: 34160024311B
NOVAPLUS
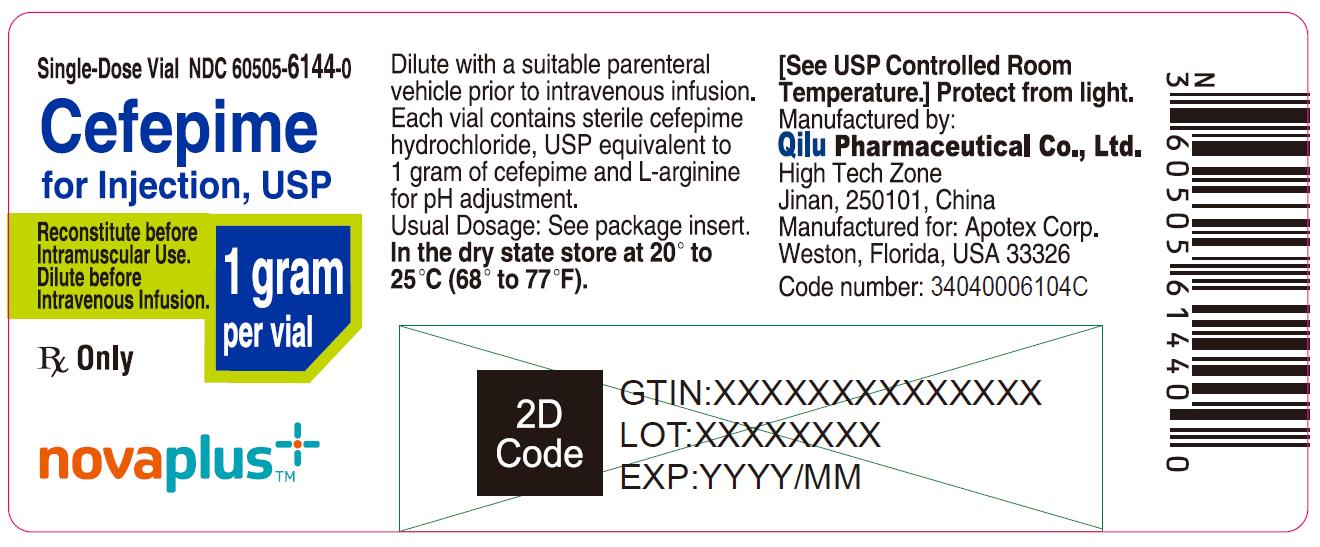
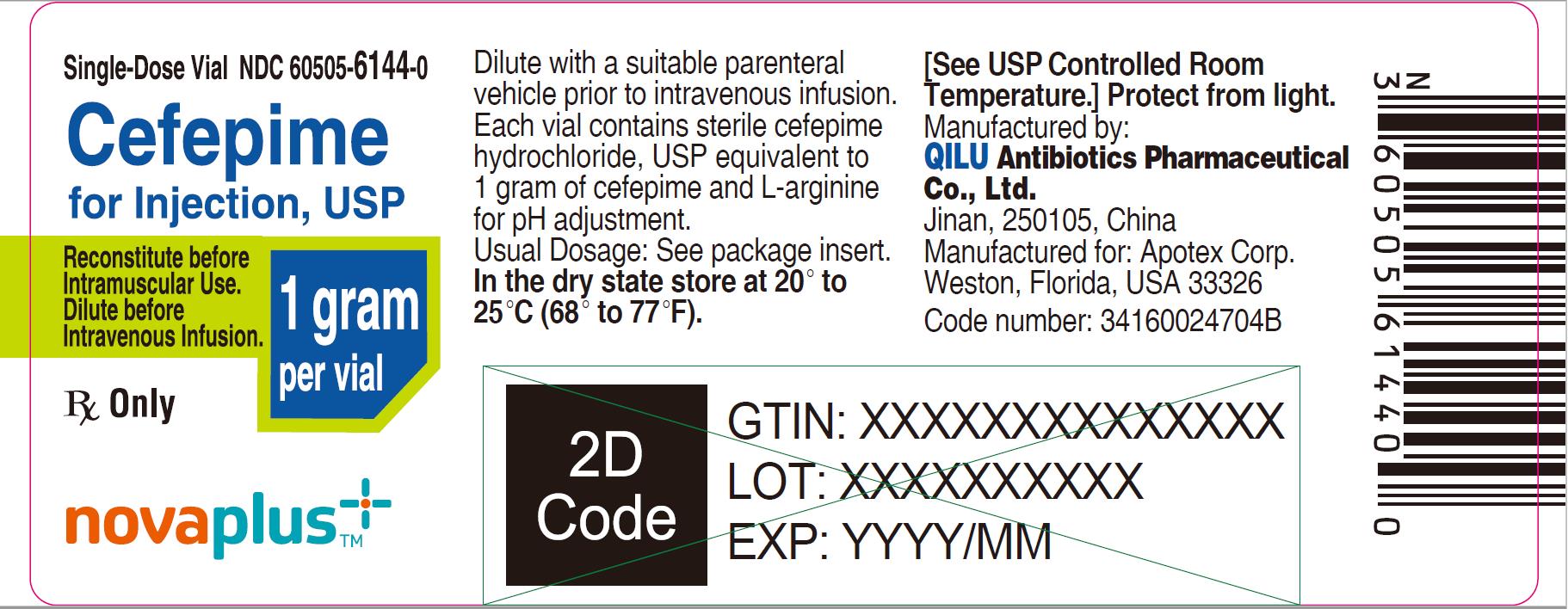
9PRINCIPAL DISPLAY PANEL - 1 gram Vial
Single-Dose Vial NDC 60505-6144-0
Cefepime for Injection, USP
1 gram per vial
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
Rx Only
NOVAPLUS
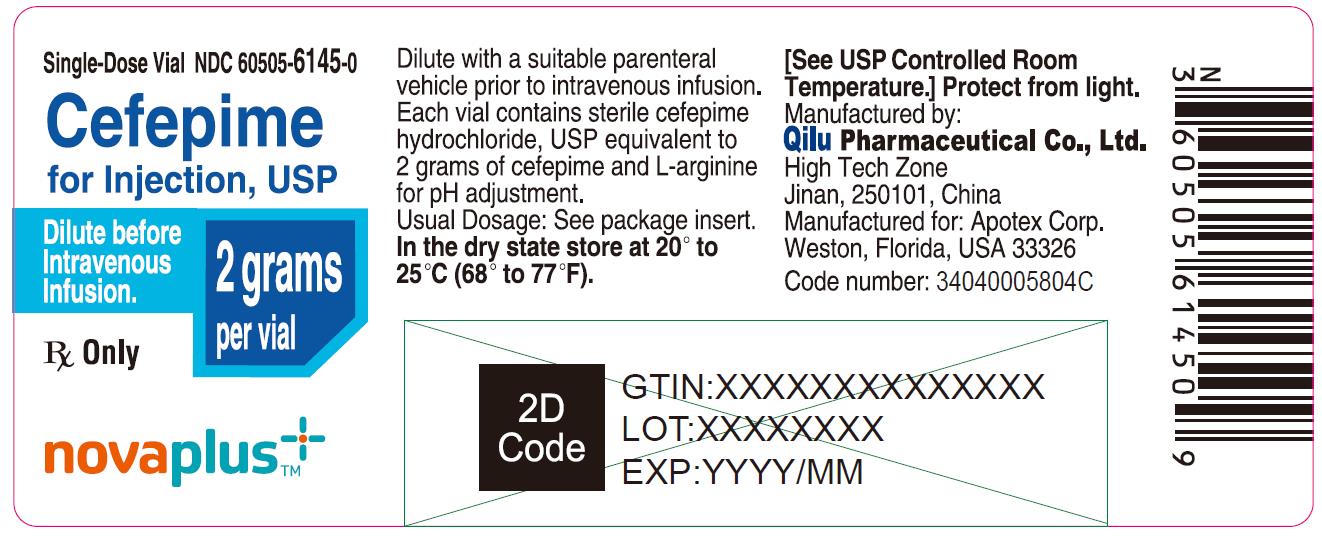
10PRINCIPAL DISPLAY PANEL - 2 gram Vial
Single-Dose Vial NDC 60505-6145-0
Cefepime for Injection, USP
2 grams per vial
Dilute before Intravenous Infusion.
Rx Only
NOVAPLUS

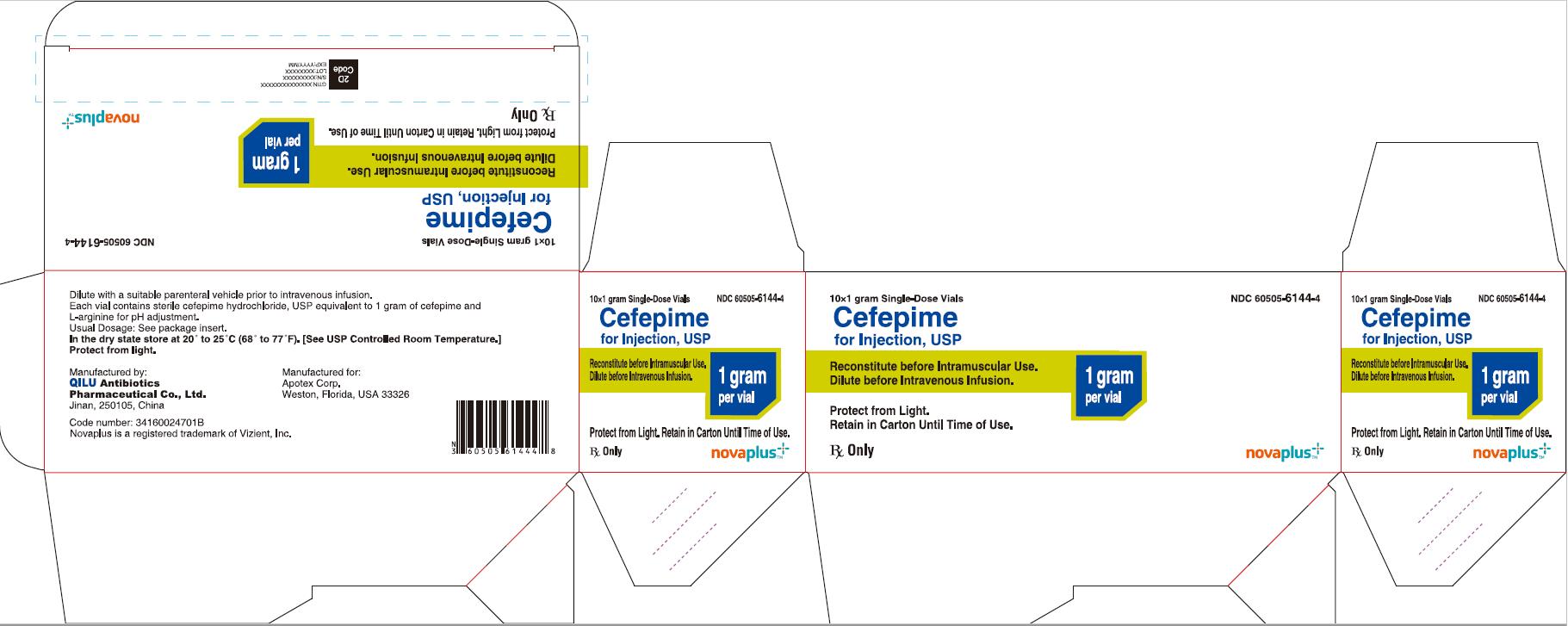
11PRINCIPAL DISPLAY PANEL - 1 gram Carton
10*1 gram Single-Dose Vials NDC 60505-6144-4
Cefepime for Injection, USP
1 gram per vial
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
Rx Only
NOVAPLUS

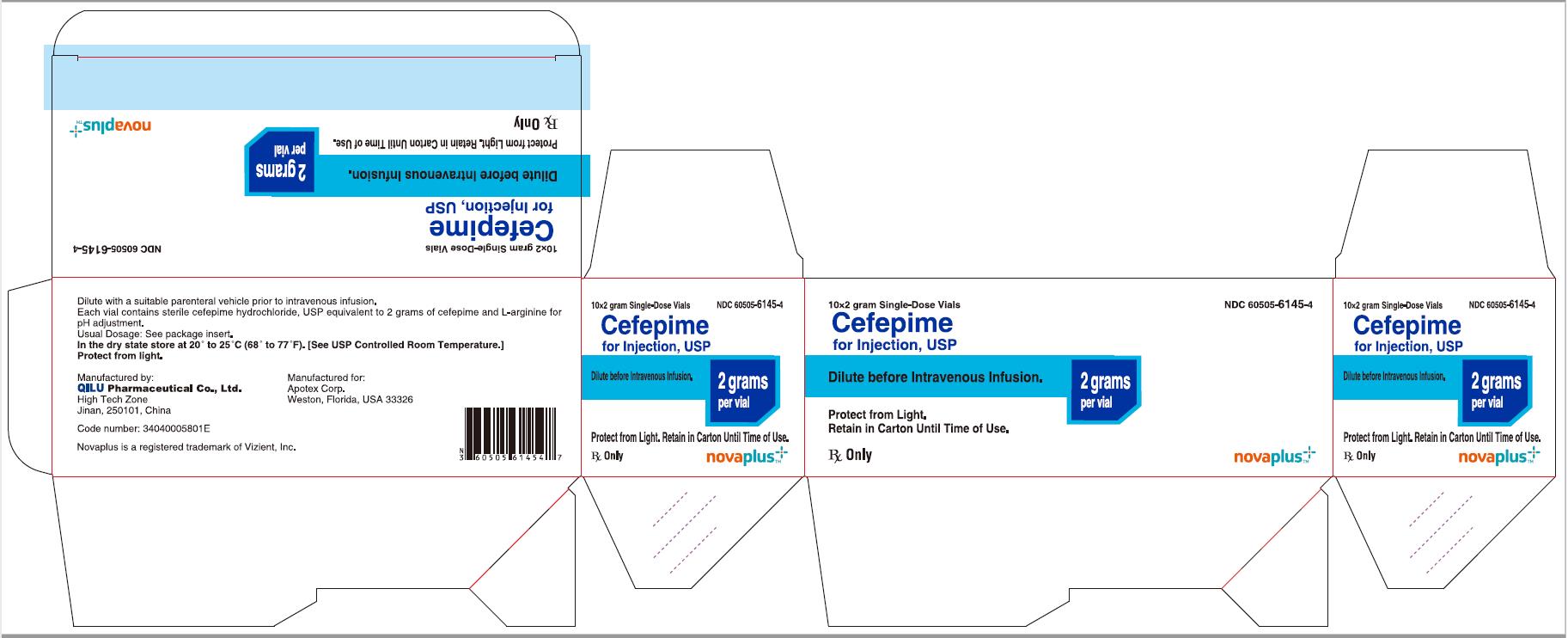
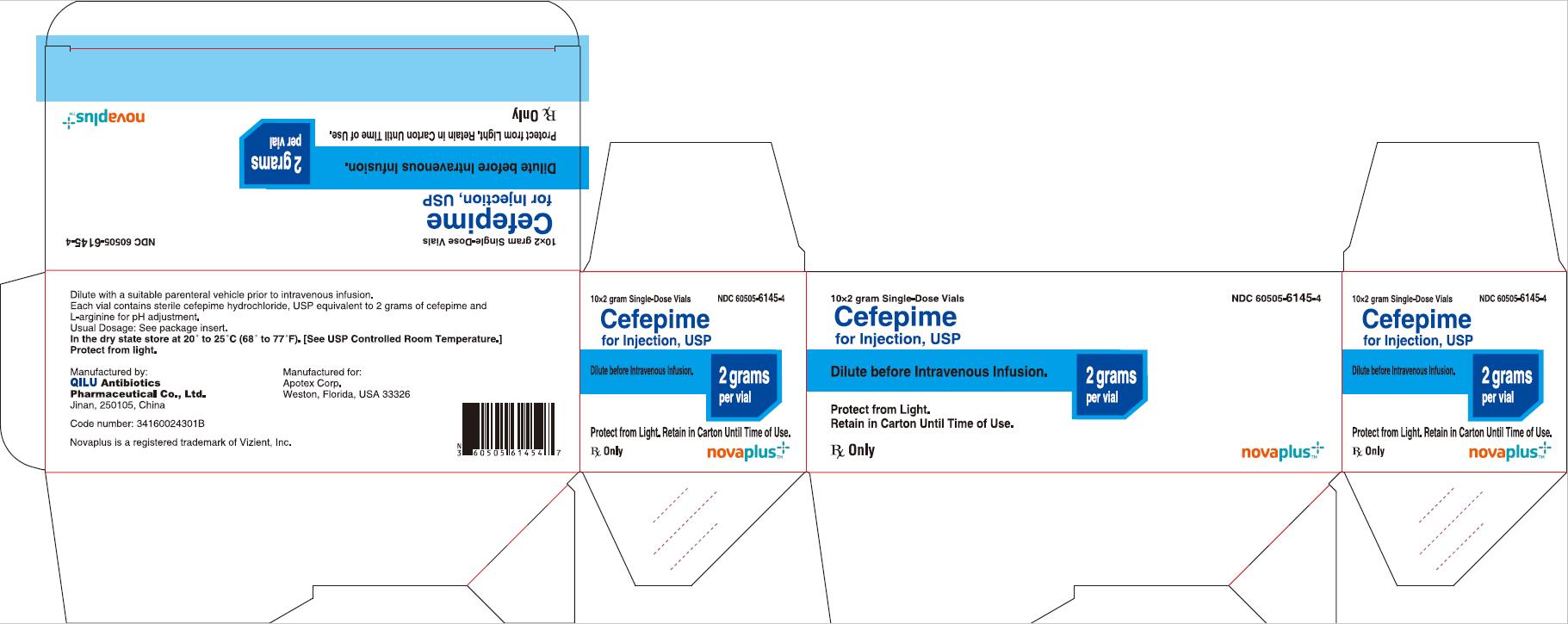
12PRINCIPAL DISPLAY PANEL - 2 gram Carton
10*2 gram Single-Dose Vials NDC 60505-6145-4
Cefepime for Injection, USP
2 grams per vial
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
Rx Only
NOVAPLUS