Generic Name
Isosorbide
Brand Names
Isordil Titradose, BiDil
FDA approval date: December 01, 1978
Classification: Nitrate Vasodilator
Form: Tablet
What is Isordil Titradose (Isosorbide)?
Isosorbide dinitrate tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Isordil (isosorbide dinitrate)
1DESCRIPTION
Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5-dinitrate, an organic nitrate whose structural formula is
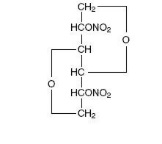
and whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins.
Isosorbide dinitrate is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of 70°C and has an optical rotation of +134° (c=1.0, alcohol, 20°C). Isosorbide dinitrate is freely soluble in organic solvents such as acetone, alcohol, and ether, but is only sparingly soluble in water.
Each Isordil® Titradose® tablet contains 5 mg or 40 mg of isosorbide dinitrate. The inactive ingredients in 5 mg tablet are magnesium stearate, lactose monohydrate, microcrystalline cellulose and FD&C red 40. The inactive ingredients in 40 mg tablet are magnesium stearate, microcrystalline cellulose, FD&C yellow 6, D&C yellow 10, and FD&C blue.
2CLINICAL PHARMACOLOGY
The principal pharmacological action of isosorbide dinitrate is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined.
Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of continuously delivered nitrates. In the large majority of these trials, active agents were no more effective than placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours has their anti-anginal efficacy been restored.
2.1Pharmacokinetics
Absorption of isosorbide dinitrate after oral dosing is nearly complete, but bioavailability is highly variable (10% to 90%), with extensive first-pass metabolism in the liver. Serum levels reach their maxima about an hour after ingestion. The average bioavailability of ISDN is about 25%; most studies have observed progressive increases in bioavailability during chronic therapy.
Once absorbed, the volume of distribution of isosorbide dinitrate is 2 to 4 L/kg, and this volume is cleared at the rate of 2 to 4 L/min, so ISDN's half-life in serum is about an hour. Since the clearance exceeds hepatic blood flow, considerable extra hepatic metabolism must also occur. Clearance is affected primarily by denitration to the 2-mononitrate (15% to 25%) and the 5-mononitrate (75% to 85%).
Both metabolites have biological activity, especially the 5-mononitrate. With an overall half-life of about 5 hours, the 5-mononitrate is cleared from the serum by denitration to isosorbide, glucuronidation to the 5-mononitrate glucuronide, and denitration/hydration to sorbitol. The 2-mononitrate has been less well studied, but it appears to participate in the same metabolic pathways, with a half-life of about 2 hours.
The daily dose-free interval sufficient to avoid tolerance to organic nitrates has not been well defined. Studies of nitroglycerin (an organic nitrate with a very short half-life) have shown that daily dose-free intervals of 10 to 12 hours are usually sufficient to minimize tolerance. Daily dose-free intervals that have succeeded in avoiding tolerance during trials of moderate doses (e.g., 30 mg) of immediate-release ISDN have generally been somewhat longer (at least 14 hours), but this is consistent with the longer half-lives of ISDN and its active metabolites.
Few well-controlled clinical trials of organic nitrates have been designed to detect rebound or withdrawal effects. In one such trial, however, subjects receiving nitroglycerin had
2.2Clinical Trials
In clinical trials, immediate-release oral isosorbide dinitrate has been administered in a variety of regimens, with total daily doses ranging from 30 mg to 480 mg. Controlled trials of single oral doses of isosorbide dinitrate have demonstrated effective reductions in exercise-related angina for up to 8 hours. Anti-anginal activity is present about 1 hour after dosing.
Most controlled trials of multiple-dose oral ISDN taken every 12 hours (or more frequently) for several weeks have shown statistically significant anti-anginal efficacy for only 2 hours after dosing. Once-daily regimens, and regimens with one daily dose-free interval of at least 14 hours (e.g., a regimen providing doses at 8 am, 2 pm, and 6 pm), have shown efficacy after the first dose of each day that was similar to that shown in the single-dose studies cited above. The effects of the second and later doses have been smaller and shorter-lasting than the effect of the first.
From large, well-controlled studies of other nitrates, it is reasonable to believe that the maximal achievable daily duration of anti-anginal effect from isosorbide dinitrate is about 12 hours. No dosing regimen for isosorbide dinitrate, however, has ever actually been shown to achieve this duration of effect. One study of 8 patients, who were administered a pretitrated dose (average 27.5 mg) of immediate-release ISDN at 8 am, 1 pm, and 6 pm for 2 weeks, revealed that significant anti-anginal effectiveness was discontinuous and totaled about 6 hours in a 24-hour period.
3INDICATIONS AND USAGE
Isordil (isosorbide dinitrate) Titradose tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
4CONTRAINDICATIONS
Isordil Titradose is contraindicated in patients who are allergic to isosorbide dinitrate or any of its ingredients.
Do not use Isordil Titradose in patients who are taking certain drugs for erectile dysfunction (phosphodiesterase inhibitors), such as sildenafil, tadalafil, or vardenafil. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia.
Do not use Isordil Titradose in patients who are taking the soluble guanylate cyclase stimulator riociguat. Concomitant use can cause hypotension.
5WARNINGS
Amplification of the vasodilatory effects of Isordil by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
The benefits of immediate-release oral isosorbide dinitrate in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use isosorbide dinitrate in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia. Because the effects of oral isosorbide dinitrate are so difficult to terminate rapidly, this formulation is not recommended in these settings.
6ADVERSE REACTIONS
Adverse reactions to isosorbide dinitrate are generally dose-related, and almost all of these reactions are the result of isosorbide dinitrate's activity as a vasodilator. Headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy. Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon.
Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients. Methemoglobinemia is so infrequent at these doses that further discussion of its diagnosis and treatment is deferred (see
Data are not available to allow estimation of the frequency of adverse reactions during treatment with Isordil Titradose tablets.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
7DOSAGE AND ADMINISTRATION
As noted under
As also noted under
Large controlled studies with other nitrates suggest that no dosing regimen with Isordil Titradose tablets should be expected to provide more than about 12 hours of continuous anti-anginal efficacy per day.
As with all titratable drugs, it is important to administer the minimum dose which produces the desired clinical effect. The usual starting dose of Isordil Titradose is 5 mg to 20 mg two or three times daily. For maintenance therapy, 10 mg to 40 mg two or three times daily is recommended. Some patients may require higher doses. A daily dose-free interval of at least 14 hours is advisable to minimize tolerance. The optimal interval will vary with the individual patient, dose and regimen.
8HOW SUPPLIED
IsordilTitradose (isosorbide dinitrate) tablets are available as follows:
5 mg, round, pink tablets imprinted "BPI 152" on one side and deeply scored on reverse side:
NDC 0187-0152-01, bottles of 100.
40 mg, round, light green tablets imprinted "BPI 192" on one side and deeply scored on reverse side:
NDC 0187-0192-01, bottles of 100.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light.
Keep bottles tightly closed.
Dispense in a light-resistant, tight container.
Keep out of reach of children
Manufactured for:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Steinbach, MB R5G 1Z7, Canada
Bausch Health Companies Inc.
Steinbach, MB R5G 1Z7, Canada
®/™ are trademarks of Bausch Health Companies Inc. or its affiliates.
© 2019 Bausch Health Companies Inc. or its affiliates
9651602 20002689
Rev. 08/2019
9PRINCIPAL DISPLAY PANEL - 5 mg Label
NDC 0187-0152-01
Rx only
Isordil
TITRADOSE®
(isosorbide dinitrate)
TITRADOSE®
(isosorbide dinitrate)
5 mg
100 Tablets
BAUSCH Health

10PRINCIPAL DISPLAY PANEL - 40 mg Label
NDC 0187-0192-01
Rx only
Isordil
TITRADOSE®
(isosorbide dinitrate)
TITRADOSE®
(isosorbide dinitrate)
40 mg
100 Tablets
Contains FD&C Yellow No. 6 as a color additive.
BAUSCH Health
