Cabergoline
View Brand InformationWhat is Cabergoline?
Approved To Treat
Related Clinical Trials
Summary: Does combined Cabergoline and letrozole increase ovulation rate What medical problems do participants have when taking drug Cabergoline and Letrozole ? Researchers will compare drug combination of Cabergoline and letrozole to letrozole alone to see combined cabergoline and letrozole works better as ovulation induction. Participants will: experimental group will receive Cabergoline and Letrozole co...
Summary: The goal of this randomized, placebo-controlled, double-blind clinical trial is to evaluate the efficacy, safety, and tolerability of cabergoline for the prevention of episodic migraine in adults with 4-14 monthly migraine days (MMD). The main questions it aims to answer are: 1. Does once-weekly cabergoline (0.5 mg or 1.0 mg) reduce MMD compared to placebo? 2. What are the effects of cabergoline o...
Summary: A Phase III clinical study evaluating the efficacy and safety of cabergoline tablets versus bromocriptine mesylate tablets in patients with hyperprolactinemia
Related Latest Advances
Brand Information
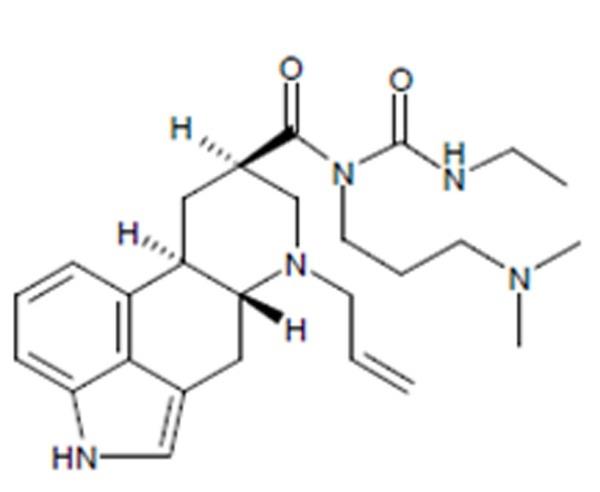
- Uncontrolled hypertension or known hypersensitivity to ergot derivatives.
- History of cardiac valvular disorders, as suggested by anatomical evidence of valvulopathy of any valve, determined by pre-treatment evaluation including echocardiographic demonstration of valve leaflet thickening, valve restriction, or mixed valve restriction‑stenosis. (See
- History of pulmonary, pericardial, or retroperitoneal fibrotic disorders. (See
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.