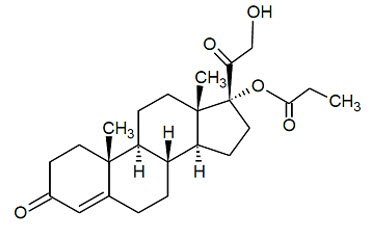
Winlevi
What is Winlevi (Clascoterone)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The primary objective of this study is to determine the safety and efficacy of clascoterone cream, 1%, versus the vehicle cream applied twice daily for 12 weeks in subjects with facial acne vulgaris.
Summary: Mechanism-based acne treatment for transgender patients receiving testosterone currently does not exist and is an unmet medical need. This study explores clascoterone to treat testosterone induced acne. Many treatments we use to treat acne in females cannot be used in transgender males because they interfere with hormone therapy. Androgens have been associated with the development of acne vulgaris...
Summary: To demonstrate the efficacy of Clascoterone cream 1% in reducing the size of sebaceous glands in study participants with acneiform rosacea.
Related Latest Advances
Brand Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Avoid applying WINLEVI Cream to damaged skin (such as cuts, abrasions), eczematous areas, and sunburned skin.
- Avoid concomitant use of other potentially irritating topical products (medicated or not).