Brand Name
Fotivda
Generic Name
Tivozanib
View Brand Information FDA approval date: March 10, 2021
Classification: Kinase Inhibitor
Form: Capsule
What is Fotivda (Tivozanib)?
FOTIVDA is indicated for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma following two or more prior systemic therapies. FOTIVDA is a kinase inhibitor indicated for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma following two or more prior systemic therapies.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Phase II Study Evaluating Efficacy of Tivozanib (AV-951) in Biliary Tract Cancers
Background: Cholangiocarcinoma (CCA) is an aggressive cancer of the bile ducts. People with CCA have few treatment options and poor survival. Researchers want to see if a new drug can stop or slow CCA growth.
Short TeRm Intensified Pembrolizumab (KEytruda) and Tivozanib for High-Risk Renal Cell Carcinoma - STRIKE
Summary: This phase III trial compares the effect of adding tivozanib to standard therapy pembrolizumab versus pembrolizumab alone for the treatment of patients with high-risk renal cell carcinoma (RCC). Immunotherapy with monoclonal antibodies, such as pembrolizumab, may help the body's immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Tivozanib is in a...
Phase 2 Study of Combination Tivozanib and Nivolumab in Advanced Non-Clear Cell Renal Cell Carcinoma
Phase 2 Study of Combination Tivozanib and Nivolumab in Advanced Non-Clear Cell Renal Cell Carcinoma
Summary: To learn if giving tivozanib in combination with nivolumab can help to control advanced nccRCC.
Related Latest Advances
Brand Information
FOTIVDA (Tivozanib)
1INDICATIONS AND USAGE
FOTIVDA is indicated for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma (RCC) following two or more prior systemic therapies.
2DOSAGE FORMS AND STRENGTHS
Capsules:
- 1.34 mg: bright yellow opaque cap imprinted with "TIVZ" in dark blue ink and a bright yellow opaque body imprinted with "SD" in dark blue ink.
- 0.89 mg: dark blue opaque cap imprinted with "TIVZ" in yellow ink and a bright yellow opaque body imprinted with "LD" in dark blue ink.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are also described elsewhere in the labeling:
- Hypertension and Hypertensive Crisis
- Cardiac Failure
- Cardiac Ischemia and Arterial Thromboembolic Events
- Venous Thromboembolic Events
- Hemorrhagic Events
- Proteinuria
- Gastrointestinal Perforation and Fistula Formation
- Thyroid Dysfunction
- Risk of Impaired Wound Healing
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
4.1Clinical Trial Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The pooled safety population described in WARNINGS AND PRECAUTIONS reflects exposure to FOTIVDA administered at 1.34 mg orally once daily with or without food for 21 days on treatment followed by 7 days off treatment for a 28-day cycle in 1008 patients with advanced RCC in TIVO-3 and five other monotherapy studies. Among 1008 patients who received FOTIVDA, 52% were exposed for 6 months or longer and 34% were exposed for greater than one year.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of FOTIVDA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: Gastrointestinal perforation and pancreatitis
5OVERDOSAGE
Overdosage with FOTIVDA can cause severe hypertension and hypertensive crisis that may result in death
During clinical studies, three patients inadvertently received doses ≥ 2.68 mg (≥ 2 times the recommended dose) of FOTIVDA. One patient who received two daily doses of 8.9 mg of FOTIVDA experienced hypertensive crisis with severe hypertensive retinopathy; a second patient who received three doses of 1.34 mg in one day experienced fatal uncontrolled hypertension; and a third patient who received two doses of 1.34 mg FOTIVDA in one day experienced persistent hypertension lasting over 5 days.
There is no specific treatment or antidote for FOTIVDA overdose.
In cases of suspected overdose, withhold FOTIVDA, closely monitor patients for hypertension and hypertensive crisis and other potential adverse reactions. Immediately manage signs or symptoms of hypertension and provide other supportive care as clinically indicated.
6DESCRIPTION
Tivozanib is a kinase inhibitor. Tivozanib hydrochloride, the active ingredient, has the chemical name 1-{2-chloro-4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-3-(5-methylisoxazol-3-yl)urea hydrochloride hydrate. The molecular formula is C
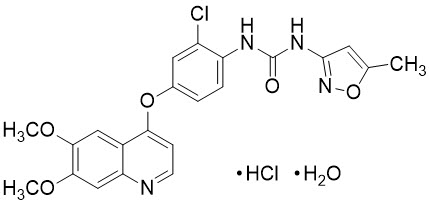
Tivozanib hydrochloride is a white to light brown crystalline powder that is practically insoluble in water (0.09 mg/mL).
FOTIVDA 1.34 mg capsule contains 1.5 mg of tivozanib hydrochloride (equivalent to 1.34 mg tivozanib) with inactive ingredients: mannitol and magnesium stearate. Capsule composition: gelatin, titanium dioxide, FDA yellow iron oxide, and Blue SB-6018 (ink).
FOTIVDA 0.89 mg capsule contains 1.0 mg of tivozanib hydrochloride (equivalent to 0.89 mg tivozanib) with inactive ingredients: mannitol and magnesium stearate. Capsule composition: gelatin, titanium dioxide, FDA yellow iron oxide, FD&C Blue #2, Blue SB-6018 (ink) and Yellow SB-3017 (ink). The Yellow SB-3017 ink contains FD&C Yellow No.5 (tartrazine).
7CLINICAL STUDIES
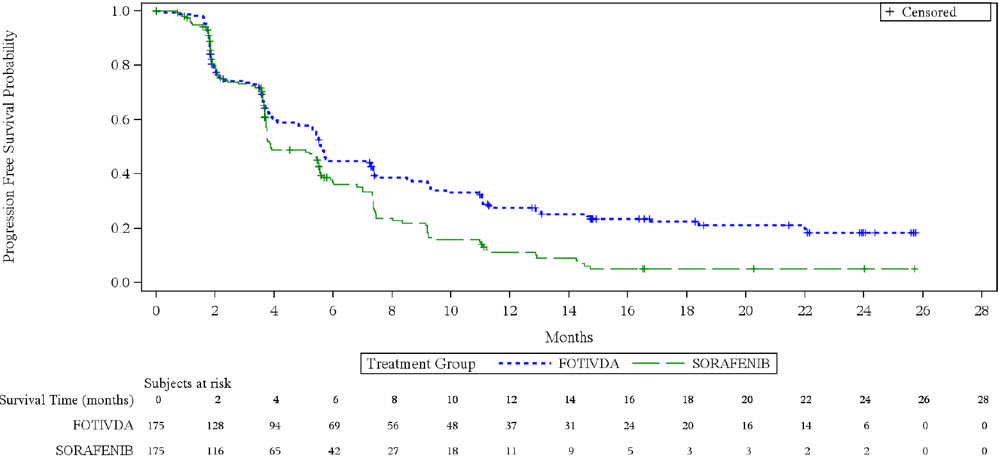
The efficacy of FOTIVDA was evaluated in TIVO-3 (NCT02627963), a randomized (1:1), open- label, multicenter trial of FOTIVDA versus sorafenib in patients with relapsed or refractory advanced RCC who received 2 or 3 prior systemic treatments including at least one VEGFR kinase inhibitor other than sorafenib or tivozanib. Patients were randomized to receive FOTIVDA 1.34 mg orally once daily for 21 days on treatment followed by 7 days off treatment for a 28-day cycle, or to receive sorafenib 400 mg orally twice a day continuously, until disease progression or unacceptable toxicity. Randomization was stratified by prior therapy [two kinase inhibitors (KIs), a KI plus an immune checkpoint inhibitor, or a KI plus other systemic agents] and by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic score. Patients were excluded if they had more than 3 prior treatments or Central Nervous System metastases. The main efficacy outcome measure was progression-free survival (PFS) assessed by a blinded independent radiology review committee. Other efficacy endpoints were objective response rate (ORR) and overall survival (OS).
The median age was 63 years (range: 30 to 90 years), 73% were male, 95% were Caucasian, ECOG performance status was 0 in 48% and 1 in 49% of patients (respectively), and 98% of patients had clear cell or clear cell component histology. Prior therapy included two KIs (45%), a KI plus an immune checkpoint inhibitor (26%), and a KI plus another systemic agent (29%). At the time of study entry, 20% of patients had favorable, 61% intermediate, and 19% poor IMDC prognoses.
Efficacy results are summarized in
Figure 1. Kaplan-Meier Plot of PFS in TIVO-3
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling
9PRINCIPAL DISPLAY PANEL - 0.89 mg Capsule Bottle Label
NDC 45629-089-02
FOTIVDA
0.89 mg
Actual Size

10PRINCIPAL DISPLAY PANEL - 1.34 mg Capsule Bottle Label
NDC 45629-134-02
FOTIVDA
1.34 mg
Actual Size


