Brand Name
Cabergoline
View Brand InformationFDA approval date: March 07, 2007
Classification: Ergot Derivative
Form: Tablet
What is Cabergoline?
Cabergoline Tablets, USP are indicated for the treatment of hyperprolactinemic disorders, either idiopathic or due to pituitary adenomas.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Cabergoline (Cabergoline)
1DESCRIPTION
Cabergoline tablets, USP contain cabergoline, USP a dopamine receptor agonist. The chemical name for cabergoline is 1-[(6-allylergolin-8β-yl)-carbonyl]-1-[3-(dimethylamino) propyl]-3-ethylurea. Its molecular formula is C
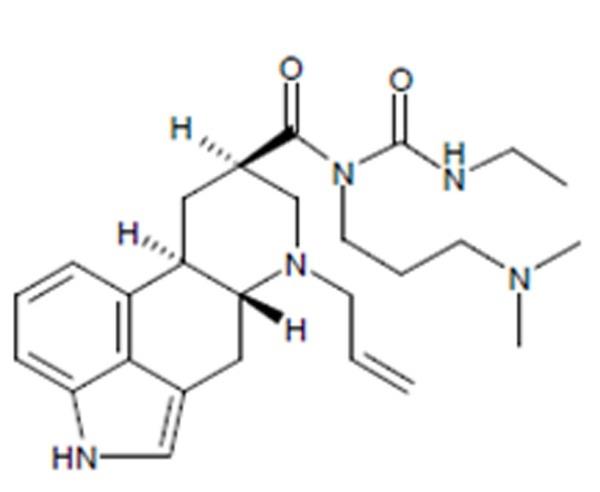
Cabergoline, USP is a white or almost white crystalline powder practically insoluble in water and hexane, very soluble in ethanol, chloroform, acetone, dichloromethane, soluble in 0.1M HCl and freely soluble in toluene.
Cabergoline tablets, USP for oral administration, contain 0.5 mg of cabergoline, USP. Inactive ingredients consist of citric acid anhydrous powder, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose.
2CLINICAL PHARMACOLOGY
Mechanism of Action
The secretion of prolactin by the anterior pituitary is mainly under hypothalamic inhibitory control, likely exerted through release of dopamine by tuberoinfundibular neurons. Cabergoline is a long-acting dopamine receptor agonist with a high affinity for D
Clinical Studies
The prolactin-lowering efficacy of cabergoline was demonstrated in hyperprolactinemic women in two randomized, double-blind, comparative studies, one with placebo and the other with bromocriptine. In the placebo-controlled study (placebo n = 20; cabergoline n = 168), cabergoline produced a dose-related decrease in serum prolactin levels with prolactin normalized after 4 weeks of treatment in 29%, 76%, 74% and 95% of the patients receiving 0.125 mg, 0.5 mg, 0.75 mg, and 1 mg twice weekly respectively.
In the 8-week, double-blind period of the comparative trial with bromocriptine (cabergoline n = 223; bromocriptine n = 236 in the intent-to-treat analysis), prolactin was normalized in 77% of the patients treated with cabergoline at 0.5 mg twice weekly compared with 59% of those treated with bromocriptine at 2.5 mg twice daily. Restoration of menses occurred in 77% of the women treated with cabergoline, compared with 70% of those treated with bromocriptine. Among patients with galactorrhea, this symptom disappeared in 73% of those treated with cabergoline compared with 56% of those treated with bromocriptine.
2.1Pharmacokinetics
Absorption
Following single oral doses of 0.5 mg to 1.5 mg given to 12 healthy adult volunteers, mean peak plasma levels of 30 picograms (pg)/mL to 70 picograms (pg)/mL of cabergoline were observed within 2 hours to 3 hours. Over the 0.5 mg to 7 mg dose range, cabergoline plasma levels appeared to be dose-proportional in 12 healthy adult volunteers and nine adult parkinsonian patients. A repeat-dose study in 12 healthy volunteers suggests that steady-state levels following a once-weekly dosing schedule are expected to be twofold to threefold higher than after a single dose. The absolute bioavailability of cabergoline is unknown. A significant fraction of the administered dose undergoes a first-pass effect. The elimination half-life of cabergoline estimated from urinary data of 12 healthy subjects ranged between 63 hours to 69 hours. The prolonged prolactin-lowering effect of cabergoline may be related to its slow elimination and long half‑life.
Distribution
In animals, based on total radioactivity, cabergoline (and/or its metabolites) has shown extensive tissue distribution. Radioactivity in the pituitary exceeded that in plasma by > 100‑fold and was eliminated with a half-life of approximately 60 hours. This finding is consistent with the long-lasting prolactin-lowering effect of the drug. Whole body autoradiography studies in pregnant rats showed no fetal uptake but high levels in the uterine wall. Significant radioactivity (parent plus metabolites) detected in the milk of lactating rats suggests a potential for exposure to nursing infants. The drug is extensively distributed throughout the body. Cabergoline is moderately bound (40% to 42%) to human plasma proteins in a concentration-independent manner. Concomitant dosing of highly protein-bound drugs is unlikely to affect its disposition.
Metabolism
In both animals and humans, cabergoline is extensively metabolized, predominately via hydrolysis of the acylurea bond or the urea moiety. Cytochrome P-450 mediated metabolism appears to be minimal. Cabergoline does not cause enzyme induction and/or inhibition in the rat. Hydrolysis of the acylurea or urea moiety abolishes the prolactin-lowering effect of cabergoline, and major metabolites identified thus far do not contribute to the therapeutic effect.
Excretion
After oral dosing of radioactive cabergoline to five healthy volunteers, approximately 22% and 60% of the dose was excreted within 20 days in the urine and feces, respectively. Less than 4% of the dose was excreted unchanged in the urine. Nonrenal and renal clearances for cabergoline are about 3.2 L/min and 0.08 L/min, respectively. Urinary excretion in hyperprolactinemic patients was similar.
Special Populations
Renal Insufficiency
The pharmacokinetics of cabergoline were not altered in 12 patients with moderate-to-severe renal insufficiency as assessed by creatinine clearance.
Hepatic Insufficiency
In 12 patients with mild-to-moderate hepatic dysfunction (Child-Pugh score ≤ 10), no effect on mean cabergoline C
Elderly
Effect of age on the pharmacokinetics of cabergoline has not been studied.
Food-Drug Interaction
In 12 healthy adult volunteers, food did not alter cabergoline kinetics.
2.2Pharmacodynamics
Dose response with inhibition of plasma prolactin, onset of maximal effect, and duration of effect has been documented following single cabergoline doses to healthy volunteers (0.05 mg to 1.5 mg) and hyperprolactinemic patients (0.3 mg to 1 mg). In volunteers, prolactin inhibition was evident at doses > 0.2 mg, while doses ≥ 0.5 mg caused maximal suppression in most subjects. Higher doses produce prolactin suppression in a greater proportion of subjects and with an earlier onset and longer duration of action. In 12 healthy volunteers, 0.5 mg, 1 mg, and 1.5 mg doses resulted in complete prolactin inhibition, with a maximum effect within 3 hours in 92% to 100% of subjects after the 1 mg and 1.5 mg doses compared with 50% of subjects after the 0.5 mg dose.
In hyperprolactinemic patients (N = 51), the maximal prolactin decrease after a 0.6 mg single dose of cabergoline was comparable to 2.5 mg bromocriptine; however, the duration of effect was markedly longer (14 days vs. 24 hours). The time to maximal effect was shorter for bromocriptine than cabergoline (6 hours vs. 48 hours).
In 72 healthy volunteers, single or multiple doses (up to 2 mg) of cabergoline resulted in selective inhibition of prolactin with no apparent effect on other anterior pituitary hormones (GH, FSH, LH, ACTH, and TSH) or cortisol.
3INDICATIONS AND USAGE
Cabergoline tablets are indicated for the treatment of hyperprolactinemic disorders, either idiopathic or due to pituitary adenomas.
4CONTRAINDICATIONS
Cabergoline tablets are contraindicated in patients with:
- Uncontrolled hypertension or known hypersensitivity to ergot derivatives.
- History of cardiac valvular disorders, as suggested by anatomical evidence of valvulopathy of any valve, determined by pre-treatment evaluation including echocardiographic demonstration of valve leaflet thickening, valve restriction, or mixed valve restriction‑stenosis. (See
- History of pulmonary, pericardial, or retroperitoneal fibrotic disorders. (See
5WARNINGS
1. Pregnancy
Dopamine agonists in general should not be used in patients with pregnancy-induced hypertension, for example, preeclampsia, eclampsia, and postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.
2.
a. Cardiac Valvulopathy
All patients should undergo a cardiovascular evaluation, including echocardiogram to assess the potential presence of valvular disease. If valvular disease is detected, the patient should not be treated with cabergoline. (See
A multi-country, retrospective cohort study using general practice records and record linkage systems in the UK, Italy and the Netherlands was conducted to assess the association between new use of dopamine agonists including cabergoline (n = 27,812) for Parkinson’s disease and hyperprolactinemia and cardiac valvular regurgitation (CVR), other fibroses, and other cardiopulmonary events over a maximum of 12 years of follow up. In this study, the use of cabergoline among persons with Parkinson's disease was associated with an increased risk of CVR when compared to non-ergot-derived dopamine agonists (DAs) and levodopa [Incidence Rate (IR) per 10,000 person years of 68.1 (95% confidence interval (CI): 37.2 to 115.3) for cabergoline vs. 10 (95% CI: 5.2 to 19.4) for non-ergot DAs and 11.3 (95% CI: 7.2 to 17) for levodopa]. In the study analysis confined to persons with dopamine agonist-treated hyperprolactinemia (n = 8,386), when compared to non-use (n = 15,147), persons exposed to cabergoline did not have an elevated risk of CVR. The findings with respect to the risk of CVR associated with cabergoline treatment for persons with Parkinson’s disease (increased risk) and those with hyperprolactinemia (no increased risk) are consistent with the findings in other published studies.
Physicians should use the lowest effective dose of cabergoline for the treatment of hyperprolactinemic disorders and should periodically reassess the need for continuing therapy with cabergoline. Following treatment initiation, clinical and diagnostic monitoring (for example, chest x-ray, CT scan and cardiac echocardiogram) should be conducted to assess the risk of cardiac valvulopathy. The recommended frequency of routine echocardiographic monitoring is every 6 months to 12 months or as clinically indicated with the presence of signs and symptoms such as edema, new cardiac murmur, dyspnea, or congestive heart failure.
Cabergoline should be discontinued if an echocardiogram reveals new valvular regurgitation, valvular restriction or valve leaflet thickening.
Cabergoline should be used with caution in patients exposed to other medications associated with valvulopathy.
b. Extracardiac Fibrotic Reactions
Postmarketing cases of pleural, pericardial, and retroperitoneal fibrosis have been reported following administration of cabergoline. Some reports were in patients previously treated with other ergotinic dopamine agonists. Cabergoline should not be used in patients with a history of cardiac or extracardiac fibrotic disorders.
Fibrotic disorders can have an insidious onset and patients should be monitored for manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of:
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.
Clinical and diagnostic monitoring such as erythrocyte sedimentation rate, chest-x ray, serum creatinine measurements, and other investigations should be considered at baseline and as necessary while patients are treated with cabergoline.
Following diagnosis of pleural effusion or pulmonary fibrosis, the discontinuance of cabergoline was reported to result in improvement of signs and symptoms.
6ADVERSE REACTIONS
The safety of cabergoline tablets has been evaluated in more than 900 patients with hyperprolactinemic disorders. Most adverse events were mild or moderate in severity.
In a 4-week, double-blind, placebo-controlled study, treatment consisted of placebo or cabergoline at fixed doses of 0.125 mg, 0.5 mg, 0.75 mg, or 1 mg twice weekly. Doses were halved during the first week. Since a possible dose-related effect was observed for nausea only, the four cabergoline treatment groups have been combined. The incidence of the most common adverse events during the placebo-controlled study is presented in the following table.
Incidence of Reported Adverse Events During the 4-Week, Double-Blind, Placebo‑Controlled Trial
*Reported at ≥ 1% for cabergoline
In the 8-week, double-blind period of the comparative trial with bromocriptine, cabergoline (at a dose of 0.5 mg twice weekly) was discontinued because of an adverse event in 4 of 221 patients (2%) while bromocriptine (at a dose of 2.5 mg two times a day) was discontinued in 14 of 231 patients (6%). The most common reasons for discontinuation from cabergoline were headache, nausea and vomiting (3 patients, 2 patients and 2 patients respectively); the most common reasons for discontinuation from bromocriptine were nausea, vomiting, headache, and dizziness or vertigo (10 patients, 3 patients, 3 patients, and 3 patients respectively). The incidence of the most common adverse events during the double-blind portion of the comparative trial with bromocriptine is presented in the following table.
Incidence of Reported Adverse Events During the 8-Week, Double-Blind Period of the Comparative Trial with Bromocriptine
*Reported at ≥ 1% for cabergoline
Other adverse events that were reported at an incidence of < 1% in the overall clinical studies follow.
Body As a Whole: facial edema, influenza-like symptoms, malaise
Cardiovascular System: hypotension, syncope, palpitations
Digestive System: dry mouth, flatulence, diarrhea, anorexia
Metabolic and Nutritional System: weight loss, weight gain
Nervous System: somnolence, nervousness, paresthesia, insomnia, anxiety
Respiratory System: nasal stuffiness, epistaxis
Skin and Appendages: acne, pruritus
Special Senses: abnormal vision
Urogenital System: dysmenorrhea, increased libido
The safety of cabergoline has been evaluated in approximately 1,200 patients with Parkinson’s disease in controlled and uncontrolled studies at dosages of up to 11.5 mg/day which greatly exceeds the maximum recommended dosage of cabergoline for hyperprolactinemic disorders. In addition to the adverse events that occurred in the patients with hyperprolactinemic disorders, the most common adverse events in patients with Parkinson’s disease were dyskinesia, hallucinations, confusion, and peripheral edema. Heart failure, pleural effusion, pulmonary fibrosis, and gastric or duodenal ulcer occurred rarely. One case of constrictive pericarditis has been reported.
Postmarketing Surveillance Data
The following events have been reported in association with cabergoline: cardiac valvulopathy and extracardiac fibrotic reactions, (See
Other events have been reported in association with cabergoline: impulse control/compulsive behavior symptoms, including hypersexuality, increased libido and pathological gambling (See
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals LLC at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
7OVERDOSAGE
Overdosage might be expected to produce nasal congestion, syncope, or hallucinations. Measures to support blood pressure should be taken if necessary.
8DOSAGE AND ADMINISTRATION
The recommended dosage of cabergoline tablets for initiation of therapy is 0.25 mg twice a week. Dosage may be increased by 0.25 mg twice weekly up to a dosage of 1 mg twice a week according to the patient’s serum prolactin level. Before initiating treatment, cardiovascular evaluation should be performed and echocardiography should be considered to assess for valvular disease.
Dosage increases should not occur more rapidly than every 4 weeks, so that the physician can assess the patient’s response to each dosage level. If the patient does not respond adequately, and no additional benefit is observed with higher doses, the lowest dose that achieved maximal response should be used and other therapeutic approaches considered. Patients receiving long‑term treatment with cabergoline should undergo periodic assessment of their cardiac status and echocardiography should be considered.
After a normal serum prolactin level has been maintained for 6 months, cabergoline may be discontinued, with periodic monitoring of the serum prolactin level to determine whether or when treatment with cabergoline should be reinstituted. The durability of efficacy beyond 24 months of therapy with cabergoline has not been established.
9HOW SUPPLIED
Cabergoline tablets, USP are white to off-white, oval shaped, flat face beveled edged tablets debossed with C and 5 on either side of bisect on one side and plain with partial break line on other side.
Cabergoline tablets, USP are available as follows:
Bottles of 8 tablets NDC 69238-2693-1
10STORAGE
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Dispense in original container.
Manufactured by:
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 08-2024-00
11PRINCIPAL DISPLAY PANEL