Mirtazapine
What is Remeron (Mirtazapine)?
Living with major depression can feel like being stuck under a heavy, invisible weight. The loss of interest, persistent sadness and profound fatigue can drain the color from life, making everyday tasks feel impossible. When depression also robs you of sleep and appetite, the struggle becomes even more physically and emotionally taxing. For individuals facing this specific combination of challenges, a unique antidepressant called Mirtazapine can be an especially helpful treatment.
Mirtazapine is a well-established prescription medication used to treat major depressive disorder. It belongs to a class of drugs known as tetracyclic antidepressants, which is different from the more common SSRIs (like Prozac or Zoloft). What makes Mirtazapine a distinct and valuable option is its dual effect on mood and its significant impact on promoting sleep and stimulating appetite. For this reason, it is often a first-line choice for patients with depression who are also struggling with insomnia and weight loss, offering a way to address multiple debilitating symptoms with a single medication.
What does Mirtazapine do?
Mirtazapine is approved by the U.S. Food and Drug Administration (FDA) for the treatment of Major Depressive Disorder (MDD) in adults. Its primary goal is to relieve the core emotional and physical symptoms of depression.
Patients who are prescribed Mirtazapine can expect a gradual improvement in their overall well-being. The benefits often include:
- Improved Mood: A reduction in feelings of sadness, hopelessness and worthlessness.
- Restored Interest and Pleasure: A renewed ability to enjoy activities and engage with life.
- Better Sleep: It is highly sedating, especially at lower doses, and can significantly help with insomnia, a common symptom of depression.
- Increased Appetite and Potential Weight Gain: It can effectively stimulate appetite, which is beneficial for patients who have lost weight due to their depression.
Mirtazapine quickly affects sleep, but its antidepressant effects on mood take 4-6 weeks to fully manifest. Its efficacy for MDD is well-established through clinical trials and extensive real-world use.
How does Mirtazapine work?
The symptoms of depression are believed to be linked to an imbalance of chemical messengers in the brain called neurotransmitters, especially serotonin and norepinephrine. These chemicals are crucial for regulating mood, sleep and alertness.
Mirtazapine has a unique and complex mechanism of action. It works by blocking specific receptors on nerve cells in the brain, which in turn increases the release of both serotonin and norepinephrine. Think of it as opening the gates to allow more of these “feel-good” and “energizing” chemicals to flow between brain cells, which helps to improve communication in the brain’s mood circuits and alleviate depressive symptoms.
Mirtazapine distinguishes itself from other antidepressants by strongly blocking the histamine H1 receptor, leading to significant drowsiness and sedation, like many over-the-counter sleep aids. This potent antihistamine effect effectively treats insomnia and stimulates appetite, giving Mirtazapine its unique clinical profile.
Mirtazapine side effects
Like all antidepressants, Mirtazapine can cause side effects. Many are most prominent when you first start the medication and may decrease as your body adjusts.
The most common side effects are directly related to its antihistamine properties:
- Drowsiness and Sedation
- Increased Appetite and Weight Gain
- Dry Mouth
- Constipation
- Dizziness
Mirtazapine has an FDA boxed warning for increased suicidal thoughts and behaviors in those up to 24. All patients and families must monitor for worsening depression, behavior changes, or self-harm thoughts, particularly early in treatment or after dose adjustments.
Rare but serious side effects include agranulocytosis (dangerous drop in white blood cells, contact doctor for infection signs like fever or sore throat), Serotonin Syndrome (life-threatening with other serotonin-raising medications), and activation of mania/hypomania in individuals with bipolar disorder.
You should not take Mirtazapine if you are taking a class of drugs called MAOIs. Do not drive or operate heavy machinery until you know how this medication affects you.
Mirtazapine dosage
Mirtazapine, an oral tablet, is typically taken once daily in the evening at bedtime due to its sedating effects. An orally disintegrating tablet (ODT) form is also available for those with swallowing difficulties. Dosage starts low and may increase, with lower doses often causing stronger sedation and higher doses yielding more prominent antidepressant effects.
Routine blood monitoring is generally not needed. Your doctor will monitor your depressive symptoms, side effects, and weight during regular appointments. A complete blood count (CBC) may be ordered if infection is suspected.
Does Mirtazapine have a generic version?
Yes, Mirtazapine is widely available as a generic medication. The brand name for Mirtazapine was Remeron (and Remeron SolTab for the disintegrating tablet), but the generic is now almost exclusively used. The U.S. Food and Drug Administration ensures that generic medications are just as safe, effective, and of the same quality as their brand-name counterparts (FDA, 2021). The availability of a generic makes this an affordable treatment option.
Conclusion
Mirtazapine is a highly effective and valuable antidepressant, particularly for individuals with major depression who also suffer from insomnia and poor appetite. Its unique mechanism provides a multifaceted approach to relieving some of the most burdensome symptoms of the illness.
While the side effects of sedation and weight gain can be a challenge for some, they can be a significant benefit for others. It is a powerful medication that requires careful medical supervision, especially regarding the risk of suicidal thoughts and other rare but serious side effects. Through an open and honest partnership with your healthcare provider, you can determine if Mirtazapine is the right choice to help you on your journey toward recovery and well-being.
References
- Food and Drug Administration (FDA). (2021). Generic Drug Facts. Retrieved from https://www.fda.gov/drugs/generic-drugs/generic-drug-facts
- Mayo Clinic. (2024). Mirtazapine (Oral Route). Retrieved from https://www.mayoclinic.org/drugs-supplements/mirtazapine-oral-route/symptoms/drg-20067335
- National Institutes of Health. (2021). Mirtazapine. MedlinePlus. Retrieved from https://medlineplus.gov/druginfo/meds/a697009.html
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- 15 mg tablets: Oval, scored, yellow, with "MSD" debossed on one side and "
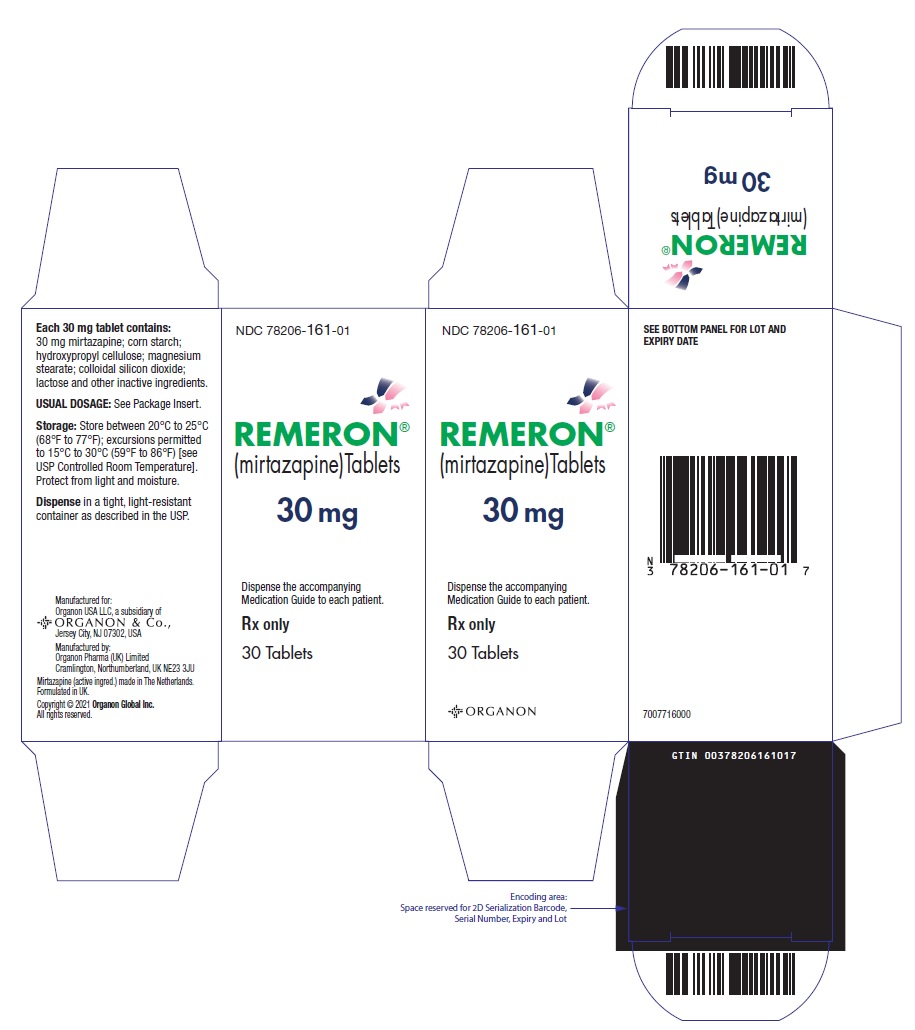
- 30 mg tablets: Oval, scored, red-brown, with "MSD" debossed on one side and "
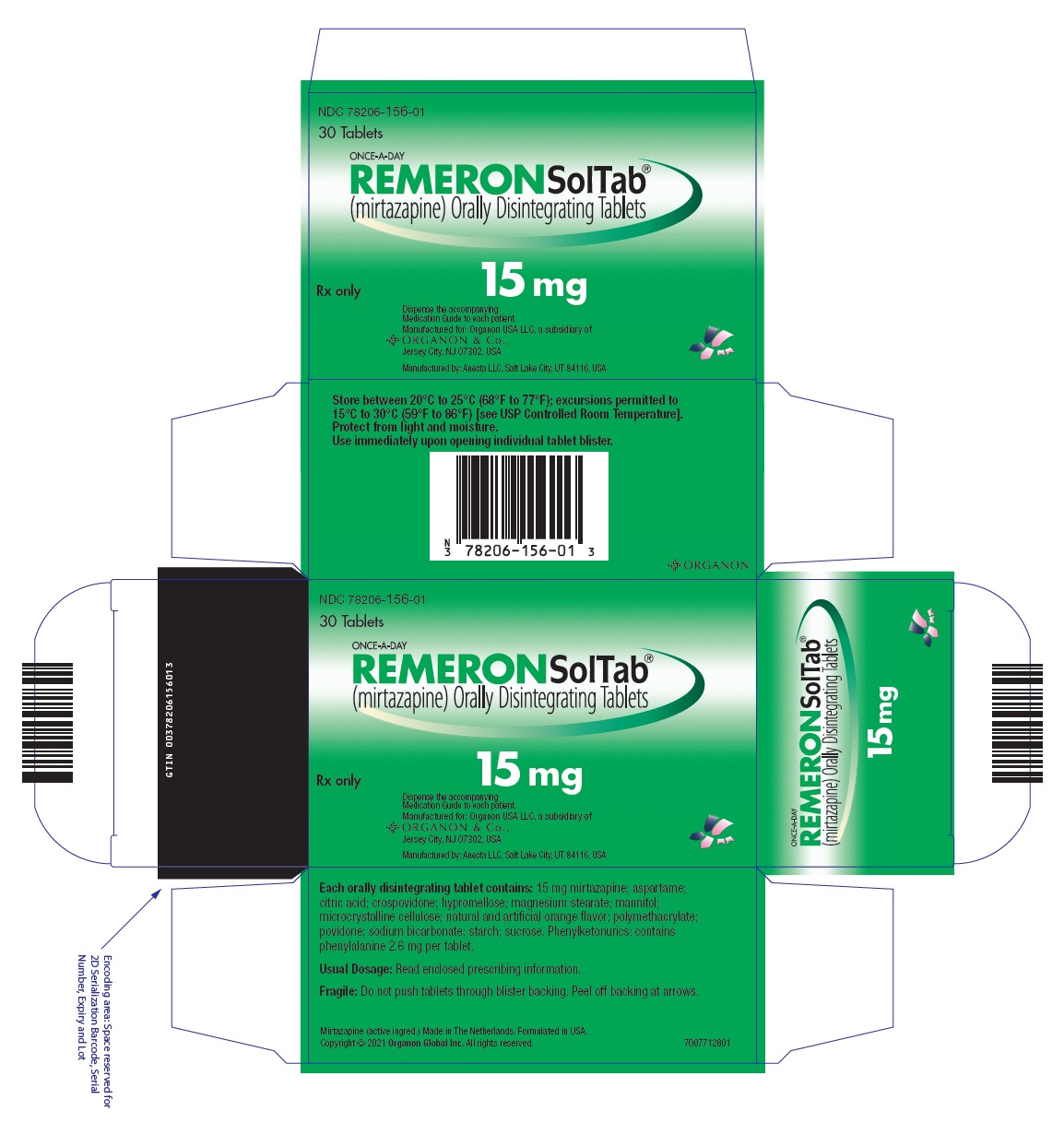
- 15 mg orally disintegrating tablets: Round, white, with "
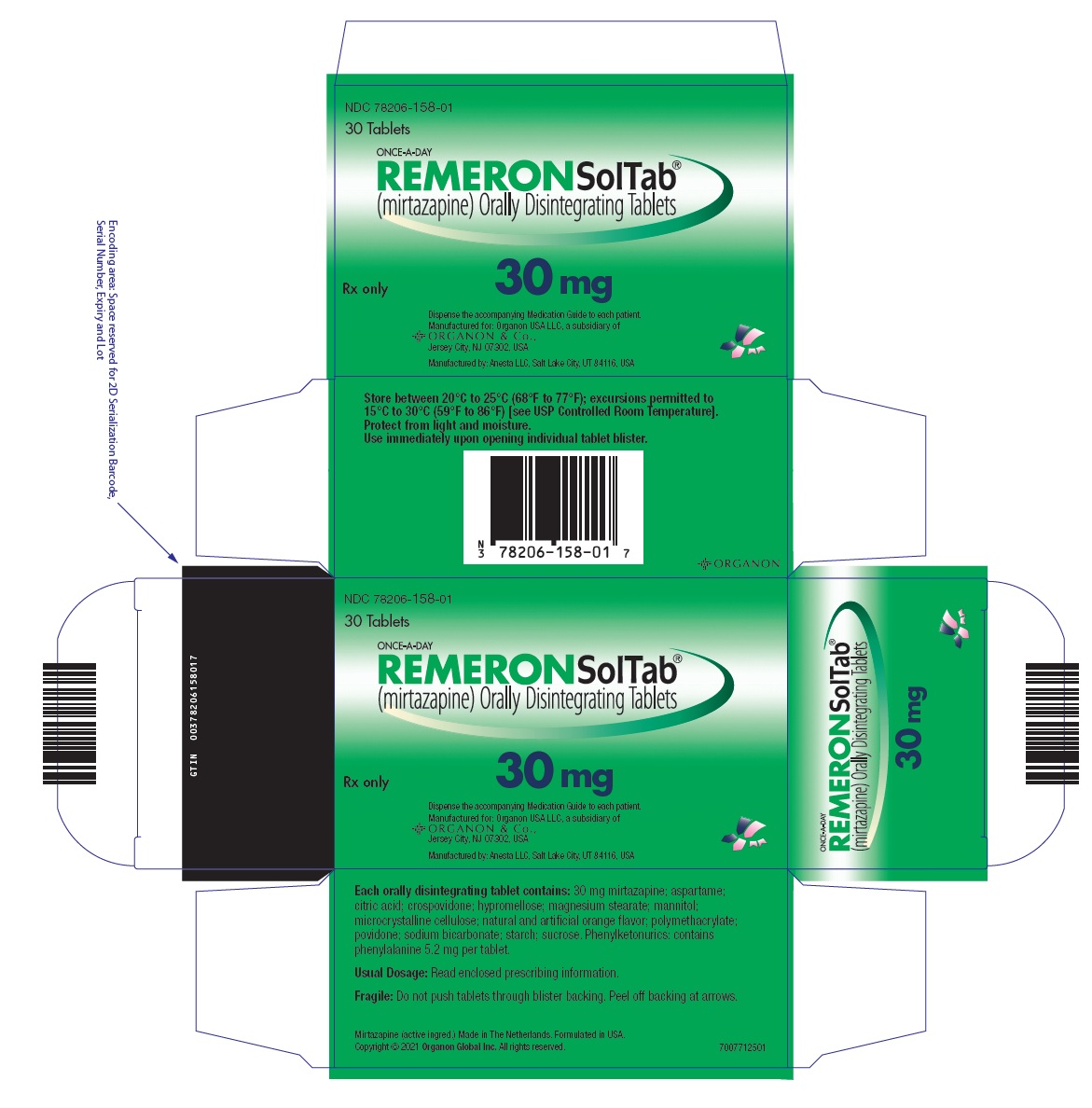
- 30 mg orally disintegrating tablets: Round, white, with "
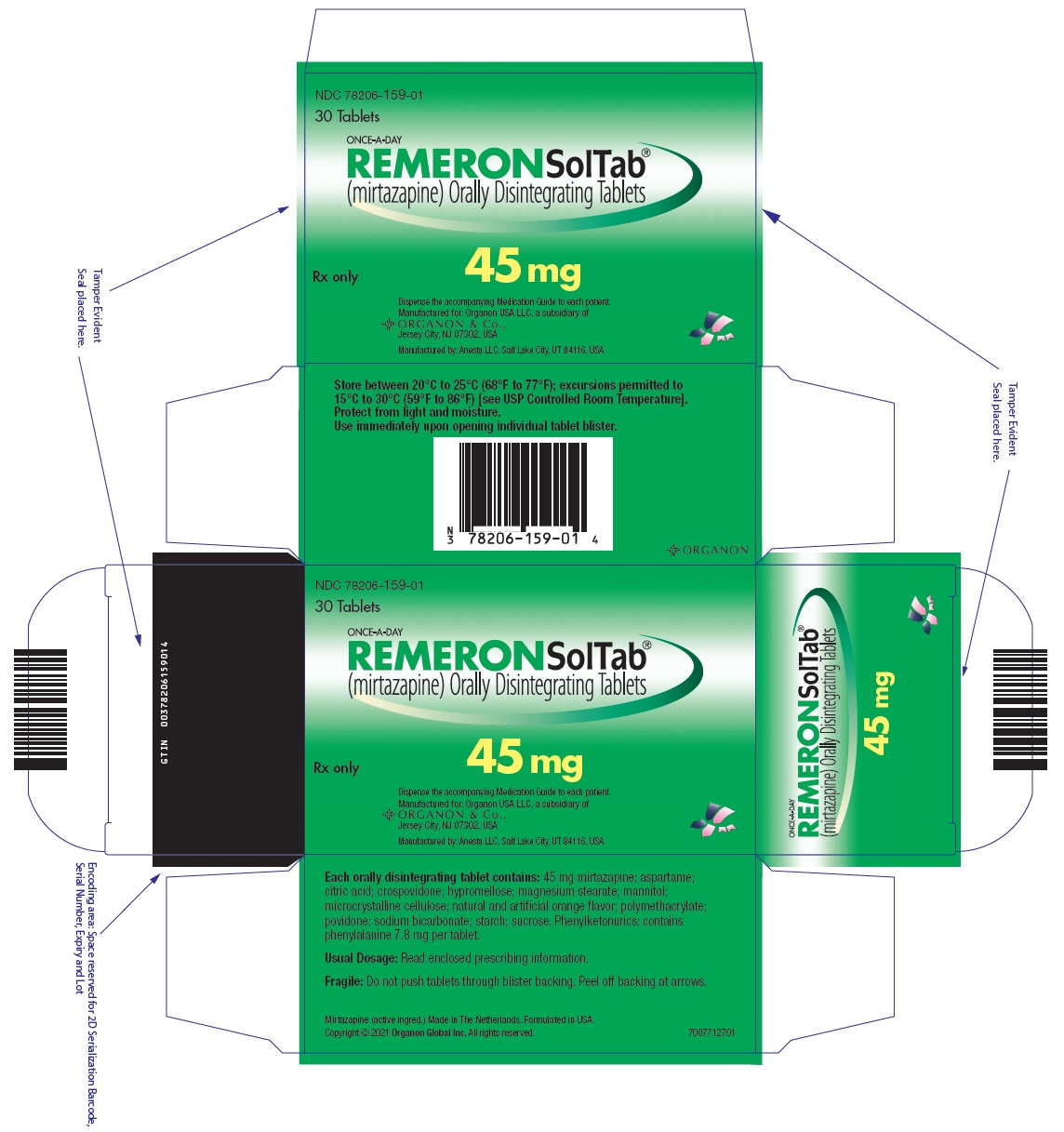
- 45 mg orally disintegrating tablets: Round, white, with "
- Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of serotonin syndrome
- With a known hypersensitivity to mirtazapine or to any of the excipients in REMERON/REMERONSolTab
- Hypersensitivity
- Suicidal Thoughts and Behaviors
- Agranulocytosis
- Serotonin Syndrome
- Angle-Closure Glaucoma
- QT Prolongation and Torsades de Pointes
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
- Increased Appetite and Weight Gain
- Somnolence
- Activation of Mania or Hypomania
- Seizures
- Elevated Cholesterol and Triglycerides
- Hyponatremia
- Transaminase Elevations
- Discontinuation Syndrome
- Use in Patients with Concomitant Illness
(mirtazapine)Tablets
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
(mirtazapine)Tablets
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.