Sutent
What is Sutent (Sunitinib)?
Receiving a cancer diagnosis can be overwhelming, but advances in targeted therapy have brought new hope for patients facing complex tumors. Sutent (generic name: sunitinib malate) is one of these important breakthroughs. It belongs to a class of medicines called tyrosine kinase inhibitors (TKIs), drugs designed to interfere with specific proteins that help cancer cells grow and spread.
Sutent is prescribed for certain cancers that are difficult to treat with traditional chemotherapy, such as kidney cancer, gastrointestinal stromal tumors (GIST) and pancreatic neuroendocrine tumors (pNET). By targeting tumor growth mechanisms directly, it offers many patients a chance for longer survival and improved quality of life.
Approved by the U.S. Food and Drug Administration (FDA) in 2006, Sutent has since become a cornerstone therapy in oncology, often used when surgery alone isn’t enough or when cancer has returned or spread to other parts of the body.
What does Sutent do?
Sutent is used to treat several specific types of cancer:
- Advanced renal cell carcinoma (RCC), a common type of kidney cancer.
- Gastrointestinal stromal tumors (GIST), cancers that start in the digestive tract, usually the stomach or small intestine, especially when other drugs like imatinib (Gleevec) no longer work.
- Pancreatic neuroendocrine tumors (pNET), rare cancers that form in hormone-producing cells of the pancreas.
For many patients, Sutent helps slow tumor growth, shrink existing tumors and delay disease progression. Clinical trials have shown that patients with advanced kidney cancer treated with sunitinib had significantly longer progression-free survival compared to those receiving standard therapy (Motzer et al., 2007).
While Sutent doesn’t cure these cancers, it can extend life expectancy, reduce symptoms, and provide patients with more time and stability in their treatment journey.
How does Sutent work?
Cancer cells rely on certain enzymes, known as tyrosine kinases, to grow new blood vessels and multiply. These enzymes act like “switches,” signaling the tumor to keep feeding itself and expanding.
Sutent works by blocking multiple tyrosine kinases at once, including those involved in tumor growth, blood vessel formation (angiogenesis), and cell replication. By shutting down these pathways, Sutent effectively starves the tumor of the nutrients and oxygen it needs to grow.
This targeted approach is different from traditional chemotherapy, which attacks all rapidly dividing cells (both healthy and cancerous). As a result, Sutent often causes fewer systemic side effects and may be better tolerated over long-term treatment.
Clinically, this mechanism matters because inhibiting angiogenesis can slow the spread of cancer and make tumors more manageable or responsive to other treatments.
Sutent side effects
While Sutent offers important benefits, it can also cause side effects that range from mild to serious. Most are manageable under a doctor’s supervision but understanding them helps patients prepare and respond appropriately.
Common side effects include:
- Fatigue or weakness
- Nausea, vomiting or diarrhea
- Mouth sores
- Changes in skin color (often a yellowish tone
- Taste changes or loss of appetite
- High blood pressure
- Hand-foot syndrome (redness, swelling, or pain on palms and soles)
Less common but potentially serious side effects:
- Heart problems, including heart failure
- Liver damage
- Bleeding or clotting issues
- Low thyroid function (hypothyroidism)
- Jawbone problems (osteonecrosis)
- Delayed wound healing
Patients should immediately contact their healthcare provider if they experience chest pain, severe shortness of breath, swelling, black stools or yellowing of the eyes or skin.
Sutent should be used cautiously in people with liver or heart disease, and it is not recommended during pregnancy due to potential harm to the unborn baby.
Regular follow-up visits and lab tests help detect side effects early and adjust treatment if necessary, keeping therapy as safe and effective as possible (FDA, 2024; Mayo Clinic, 2024).
Sutent dosage
Sutent is available as oral capsules taken once daily, usually in treatment cycles that include periods of taking the medication followed by rest days to help the body recover. The exact schedule and duration depend on the cancer type, disease stage, and individual response.
Doctors closely monitor blood pressure, liver enzymes, thyroid, and heart health during therapy due to multi-organ effects. Imaging scans track tumor response. Treatment may be paused, reduced, or changed if organ function alters or severe side effects occur.
Older adults and those with pre-existing heart or liver conditions need closer monitoring. Patients should stay hydrated, avoid grapefruit, and report unusual symptoms promptly.
Does sutent have a generic version?
Yes. The FDA has approved generic versions of sunitinib malate, which are available in the United States and other countries. Generic sunitinib is bioequivalent to the brand-name Sutent, meaning it works in the same way and offers the same effectiveness and safety profile.
Using a generic version can significantly reduce treatment costs, which is especially helpful for patients requiring long-term therapy. However, some oncologists may initially prescribe the brand formulation to ensure consistent response, especially when beginning treatment.
Always check with your healthcare provider or pharmacist to confirm which formulation you are receiving and whether it is approved by the FDA or a recognized regulatory body.
Conclusion
Sutent represents a major step forward in personalized cancer care, offering hope to patients with difficult-to-treat tumors like renal cell carcinoma, GIST, and pancreatic neuroendocrine tumors. By blocking the specific pathways cancer cells use to grow and spread, it provides a more targeted and often better-tolerated alternative to traditional chemotherapy.
For many patients, Sutent has transformed what once felt like a hopeless situation into a manageable condition, allowing them to live longer and maintain a better quality of life. When prescribed and monitored by a qualified healthcare professional, Sutent offers both control and confidence in the fight against cancer.
References
- U.S. Food and Drug Administration (FDA). (2024). Sunitinib Malate (Sutent) – Prescribing Information. https://www.fda.gov/
- Mayo Clinic. (2024). Sunitinib (Oral Route): Uses, Side Effects, and Precautions. https://www.mayoclinic.org/
- Motzer, R. J., et al. (2007). Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine, 356(2), 115–124. https://www.nejm.org/
Top Global Experts
Related Clinical Trials
Summary: Main Objective of this study is to examine long-term safety of nivolumab monotherapy including combinations and other cancer therapies in various tumor types.
Summary: This is a Phase 3, 2-arm, randomized, open-label, global, multicenter study comparing the efficacy of ripretinib to sunitinib in participants with GIST who progressed on first-line treatment with imatinib, harbor co-occurring KIT exons 11+17/18 mutations, and are without KIT exon 9, 13, or 14 mutations. Upon disease progression as determined by an independent radiologic review, participants random...
Summary: This study aims to determine the safety, pharmacokinetics (PK) and recommended Phase 3 dose (RP3D) of RYZ101 in Part 1, and the safety, efficacy, and PK of RYZ101 compared with investigator-selected standard of care (SoC) therapy in Part 2 in subjects with inoperable, advanced, well-differentiated, somatostatin receptor expressing (SSTR+) gastroenteropancreatic neuroendocrine tumors (GEP-NETs) tha...
Related Latest Advances
Brand Information
- 12.5 mg sunitinib: orange cap and orange body, printed with white ink “Pfizer” on the cap and “STN 12.5 mg” on the body.
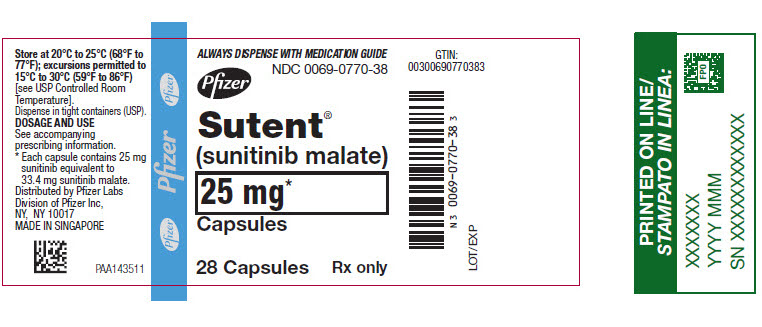
- 25 mg sunitinib: caramel cap and orange body, printed with white ink “Pfizer” on the cap and “STN 25 mg” on the body.
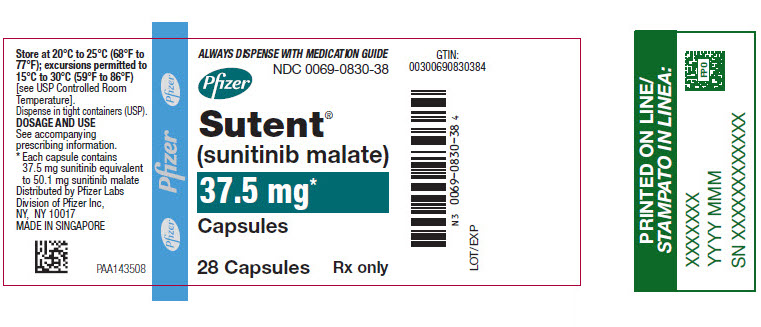
- 37.5 mg sunitinib: yellow cap and yellow body, printed with black ink “Pfizer” on the cap and “STN 37.5 mg” on the body.
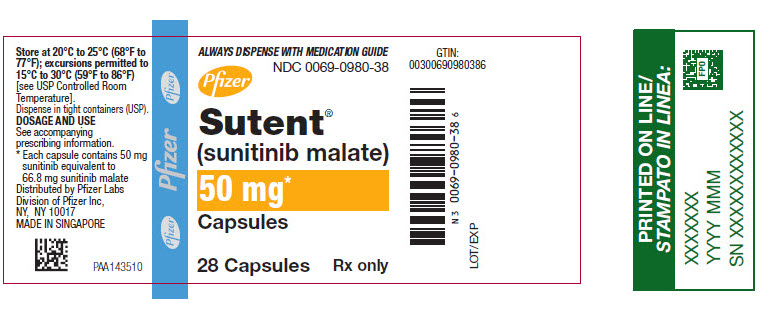
- 50 mg sunitinib: caramel top and caramel body, printed with white ink “Pfizer” on the cap and “STN 50 mg” on the body.
- Hepatotoxicity
- Cardiovascular Events
- QT Interval Prolongation and Torsade de Pointes
- Hypertension
- Hemorrhagic Events
- Tumor Lysis Syndrome
- Thrombotic Microangiopathy
- Proteinuria
- Dermatologic Toxicities
- Reversible Posterior Leukoencephalopathy Syndrome
- Thyroid Dysfunction
- Hypoglycemia
- Osteonecrosis of the Jaw
- Impaired Wound Healing
- Blood and lymphatic system disorders: hemorrhage associated with thrombocytopenia
including some fatalities . - Gastrointestinal disorders: esophagitis.
- Hepatobiliary disorders: cholecystitis, particularly acalculous cholecystitis.
- Immune system disorders: hypersensitivity reactions, including angioedema.
- Infections and infestations: serious infection (with or without neutropenia)
. The infections most commonly observed with SUTENT include respiratory, urinary tract, skin infections, and sepsis/septic shock. - Musculoskeletal and connective tissue disorders: fistula formation, sometimes associated with tumor necrosis and/or regression
; myopathy and/or rhabdomyolysis with or without acute renal failure . - Renal and urinary disorders: renal impairment and/or failure
. - Respiratory disorders: pulmonary embolism
, pleural effusion . - Skin and subcutaneous tissue disorders: pyoderma gangrenosum, including positive de-challenges.
- Vascular disorders: arterial (including aortic) aneurysms, dissections
, and rupture ; arterial thromboembolic events . The most frequent events included cerebrovascular accident, transient ischemic attack, and cerebral infarction. - General disorders and administration site conditions: impaired wound healing.