Fruzaqla
What is Fruzaqla (Fruquintinib)?
For many people living with advanced colorectal cancer, treatment often involves years of managing therapies, side effects, and uncertainty. When the disease progresses despite prior treatments, patients and their families look for new options that can extend life and improve comfort. Fruzaqla (fruquintinib) is one such option, offering hope through a modern, targeted approach to controlling tumor growth.
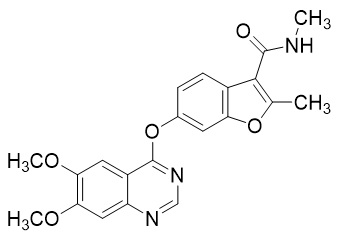
Fruzaqla is an oral cancer medication that belongs to a class of drugs known as vascular endothelial growth factor receptor (VEGFR) inhibitors. It works by blocking the blood vessels that supply nutrients to tumors, effectively slowing or stopping their growth. Developed by HUTCHMED and marketed by Takeda in the United States, Fruzaqla was approved by the U.S. Food and Drug Administration (FDA) in 2023 for the treatment of metastatic colorectal cancer (mCRC) that has progressed after standard chemotherapy and biologic therapies.
As a targeted therapy, Fruzaqla represents a newer generation of anti-cancer medications designed to specifically interfere with tumor biology, helping patients manage advanced disease while maintaining a better quality of life.
What does Fruzaqla do?
Fruzaqla is used to treat adults with metastatic colorectal cancer whose cancer has continued to grow or spread after previous treatments. Typically, patients receiving Fruzaqla have already undergone chemotherapy with drugs such as fluoropyrimidine, oxaliplatin, and irinotecan, and have tried anti-VEGF or anti-EGFR therapies.
The medication helps slow the progression of cancer by cutting off its blood supply, a process essential for tumor survival and growth. In clinical studies, Fruzaqla has been shown to extend overall survival and progression-free survival compared to placebo in patients who have exhausted other treatment options (FDA, 2023).
While Fruzaqla is not a cure, it offers a way to stabilize the disease, helping patients live longer with fewer symptoms such as pain, fatigue, and weight loss. Many patients find that this treatment helps maintain a sense of control and allows for continued daily functioning, making it a valuable option in the later stages of colorectal cancer care.
How does Fruzaqla work?
Fruzaqla (fruquintinib) works by blocking the activity of specific enzymes called VEGF receptors (VEGFR-1, VEGFR-2, and VEGFR-3) that control the formation of new blood vessels, a process known as angiogenesis.
Tumors rely on these newly formed blood vessels to deliver oxygen and nutrients, allowing them to grow and spread. By inhibiting VEGFRs, Fruzaqla starves the tumor of its blood supply, slowing or stopping its growth. This mechanism is particularly effective in advanced colorectal cancer, where abnormal blood vessel formation plays a key role in tumor progression.
In simple terms, Fruzaqla helps the body “cut off the tumor’s lifeline”, reducing its ability to thrive. Unlike traditional chemotherapy that targets rapidly dividing cells throughout the body, Fruzaqla specifically targets the signaling pathways that support tumor blood vessel growth, potentially leading to fewer systemic side effects and better tolerability for patients.
Clinically, this targeted mechanism matters because it gives doctors a precision therapy option for patients who may no longer benefit from standard chemotherapy, offering renewed hope for disease management.
Fruzaqla side effects
Like all cancer therapies, Fruzaqla can cause side effects. Most are manageable and can be monitored closely by the healthcare team.
Common side effects may include:
- High blood pressure (hypertension)
- Fatigue or weakness
- Diarrhea
- Mouth sores (stomatitis)
- Hand-foot syndrome (redness, pain, or swelling on palms and soles)
- Loss of appetite
- Abdominal pain
- Changes in liver enzyme levels
Serious side effects (less common) may include:
- Severe bleeding or blood clots
- Gastrointestinal perforation (tear in the stomach or intestines)
- Liver problems
- Heart issues such as heart failure or abnormal rhythm
- Impaired wound healing
Fruzaqla may require temporary discontinuation before and after surgery for proper healing. Patients should seek immediate medical attention for severe abdominal pain, coughing up blood, shortness of breath, chest pain, or sudden limb swelling.
Doctors will monitor blood pressure, liver function, and blood counts to manage side effects, which are generally reversible with timely intervention.
Fruzaqla dosage
Fruzaqla is an oral tablet taken once daily, usually in cycles with rest periods. Dosing is adjusted by a healthcare provider based on tolerance, lab results, and side effects.
Doctors monitor blood pressure, heart, liver, and kidney function, and blood counts during treatment. If side effects are severe, treatment may be paused or the dose lowered.
Dosing adjustments may be made for older adults or those with existing liver or kidney issues. Patients should take Fruzaqla as prescribed for optimal results.
Does Fruzaqla have a generic version?
As of 2025, Fruzaqla (fruquintinib) does not have a generic version available in the United States or internationally. It is marketed exclusively under the brand name Fruzaqla, produced by Takeda Pharmaceuticals. However, international versions may exist in other markets.
When the patent expires, generic versions of Fruzaqla, with the same active ingredient, strength, quality, and effectiveness as the brand-name medication, may become available after FDA approval. Patients struggling to afford Fruzaqla should inquire about patient assistance programs from Takeda or independent foundations.
Conclusion
Fruzaqla (fruquintinib) represents a significant advancement in the treatment of metastatic colorectal cancer, especially for patients whose disease has progressed after standard therapies. By blocking the growth of blood vessels that feed tumors, it helps control cancer spread, extend survival, and maintain quality of life.
Fruzaqla’s side effects are manageable with medical supervision and communication, ensuring safe and beneficial treatment. It offers a powerful new option for colorectal cancer patients, providing hope and time to continue their fight with strength and dignity under oncology guidance.
References
- U.S. Food and Drug Administration (FDA). (2023). FDA approves Fruzaqla (fruquintinib) for previously treated metastatic colorectal cancer. Retrieved from https://www.fda.gov
- Mayo Clinic. (2024). Fruquintinib: Uses, side effects, and safety information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Fruquintinib oral: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Cancer Institute (NCI). (2024). Targeted therapies for colorectal cancer. Retrieved from https://www.cancer.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: High blood pressure (hypertension) is a known side effect of the treatment with fruquintinib. Current research does not provide a clear answer whether minority groups such as Black/African American and/or Hispanic/Latino with refractory metastatic colorectal cancer (mCRC) have a bigger risk of higher blood pressure after treatment with fruquintinib. The main aim of this study is to learn how often...
Summary: This is a prospective, randomized, open-label, multicenter phase II investigating the therapy of Fruquintinib in combination with Tislelizumab in patients with MSS/pMMR metastatic colorectal cancer without liver metastases.
Summary: The purpose of this study is to estimate the proportion of all adverse events (AEs) including serious adverse events (SAEs) occurring with the use of fruquintinib among adult participants who have been administered fruquintinib as per the approved indications.
Related Latest Advances
Brand Information
- 1 mg: size 3 hard gelatin capsule with standard yellow opaque cap and white opaque body, imprinted with “HM013” over “1 mg” on the body in black ink.
- 5 mg: size 1 hard gelatin capsule with a red opaque cap and white opaque body, imprinted with “HM013” over “5 mg” on the body in black ink.
- Hypertension
- Hemorrhagic Events
- Infections
- Gastrointestinal Perforation
- Hepatotoxicity
- Proteinuria
- Palmar-Plantar Erythrodysesthesia (PPE)
- Posterior Reversible Encephalopathy Syndrome (PRES)
FRESCO-2 Study
The safety of FRUZAQLA was evaluated in FRESCO-2, a randomized, double-blind, placebo-controlled study [see . Patients received either FRUZAQLA 5 mg daily for the first 21 days of each 28-day cycle plus best supportive care (BSC) (n=456) or matching placebo plus BSC (n=230).
The safety of FRUZAQLA was evaluated in FRESCO, a randomized, double-blind, placebo-controlled study [see . Patients received either FRUZAQLA 5 mg daily for the first 21 days of each 28-day cycle plus BSC (n=278) or matching placebo plus BSC (n=137).
(fruquintinib) capsules
(fruquintinib) capsules

(fruquintinib) capsules
(fruquintinib) capsules



