Generic Name
Pregabalin
Brand Names
Lyrica, Pregablin
FDA approval date: December 30, 2004
Form: Tablet, Capsule, Solution
What is Lyrica (Pregabalin)?
Pregabalin capsules are indicated for: Management of neuropathic pain associated with diabetic peripheral neuropathy, Management of postherpetic neuralgia, Adjunctive therapy for the treatment of partial onset seizures in patients 17 years of age and older, Management of fibromyalgia, Management of neuropathic pain associated with spinal cord injury Pediatric use information is approved for Pfizer's LYRICA Capsules and Oral Solution products. However, due to Pfizer's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Lyrica (PREGABALIN)
1INDICATIONS AND USAGE
LYRICA is indicated for:
- Management of neuropathic pain associated with diabetic peripheral neuropathy
- Management of postherpetic neuralgia
- Adjunctive therapy for the treatment of partial onset seizures in patients 4 years of age and older
- Management of fibromyalgia
- Management of neuropathic pain associated with spinal cord injury
2DOSAGE FORMS AND STRENGTHS
Capsules: 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, and 300 mg
Oral Solution: 20 mg/mL
[see
3CONTRAINDICATIONS
LYRICA is contraindicated in patients with known hypersensitivity to pregabalin or any of its components. Angioedema and hypersensitivity reactions have occurred in patients receiving pregabalin therapy
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Angioedema
- Hypersensitivity
- Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation
- Suicidal Behavior and Ideation
- Peripheral Edema
- Dizziness and Somnolence
- Weight Gain
- Tumorigenic Potential
- Ophthalmological Effects
- Creatine Kinase Elevations
- Decreased Platelet Count
- PR Interval Prolongation
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials across various patient populations during the premarketing development of LYRICA, more than 10,000 patients have received LYRICA. Approximately 5000 patients were treated for 6 months or more, over 3100 patients were treated for 1 year or longer, and over 1400 patients were treated for at least 2 years.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LYRICA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System Disorders – Headache
Gastrointestinal Disorders – Nausea, Diarrhea
Reproductive System and Breast Disorders – Gynecomastia, Breast Enlargement
In addition, there are postmarketing reports of events related to reduced lower gastrointestinal tract function (e.g., intestinal obstruction, paralytic ileus, constipation) when LYRICA was co-administered with medications that have the potential to produce constipation, such as opioid analgesics. There are also postmarketing reports of respiratory failure and coma in patients taking pregabalin and other CNS depressant medications.
5DRUG INTERACTIONS
Since LYRICA is predominantly excreted unchanged in the urine, undergoes negligible metabolism in humans (less than 2% of a dose recovered in urine as metabolites), and does not bind to plasma proteins, its pharmacokinetics are unlikely to be affected by other agents through metabolic interactions or protein binding displacement.
6DESCRIPTION
Pregabalin is described chemically as (
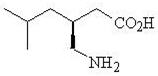
Pregabalin is a white to off-white, crystalline solid with a pK
LYRICA (pregabalin) Capsules are administered orally and are supplied as imprinted hard-shell capsules containing 25, 50, 75, 100, 150, 200, 225, and 300 mg of pregabalin, along with lactose monohydrate, cornstarch, and talc as inactive ingredients. The capsule shells contain gelatin and titanium dioxide. In addition, the orange capsule shells contain red iron oxide and the white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide. Colloidal silicon dioxide is a manufacturing aid that may or may not be present in the capsule shells. The imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide.
LYRICA (pregabalin) oral solution, 20 mg/mL, is administered orally and is supplied as a clear, colorless solution contained in a 16 fluid ounce white HDPE bottle with a polyethylene-lined closure. The oral solution contains 20 mg/mL of pregabalin, along with methylparaben, propylparaben, monobasic sodium phosphate anhydrous, dibasic sodium phosphate anhydrous, sucralose, artificial strawberry #11545 and purified water as inactive ingredients.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
8Repackaging Information
Please reference the
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:
9PRINCIPAL DISPLAY PANEL - 25 mg
NDC 71610-071 - Pregabalin (Lyrica) 25 mg - CV - Rx Only