Brand Name
Inqovi
Generic Name
Decitabine
View Brand Information FDA approval date: July 11, 2013
Classification: Nucleoside Metabolic Inhibitor
Form: Injection, Tablet, Kit
What is Inqovi (Decitabine)?
INQOVI is indicated for treatment of adult patients with myelodysplastic syndromes , including previously treated and untreated, de novo and secondary MDS with the following French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia ) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups. INQOVI is a combination of decitabine, a nucleoside metabolic inhibitor, and cedazuridine, a cytidine deaminase inhibitor, indicated for treatment of adult patients with myelodysplastic syndromes , including previously treated and untreated, de novo and secondary MDS with the following French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia ) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
INQOVI (CEDAZURIDINE and DECITABINE)
1INDICATIONS AND USAGE
INQOVI is indicated for treatment of adult patients with myelodysplastic syndromes (MDS), including previously treated and untreated, de novo and secondary MDS with the following French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia [CMML]) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.
2DOSAGE FORMS AND STRENGTHS
INQOVI tablets contain 35 mg decitabine and 100 mg cedazuridine. The tablets are biconvex, oval-shaped, film-coated, red and debossed with “H35” on one side.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression
4.1Clinical Trials Experience
Because clinical trials are conducted under widely variable conditions, adverse event rates observed in clinical trials of a drug cannot be directly compared with rates of clinical trials of another drug and may not reflect the rates observed in practice.
Myelodysplastic Syndrome and Chronic Myelomonocytic Leukemia
The safety of INQOVI was evaluated in a pooled safety population that includes patients enrolled in Study ASTX727-01-B and Study ASTX727-02
Patients were randomized to receive INQOVI (35 mg decitabine and 100 mg cedazuridine) orally once daily on Days 1 through 5 in Cycle 1 and decitabine 20 mg/m
Serious adverse reactions occurred in 68% of patients who received INQOVI. Serious adverse reactions in > 5% of patients included febrile neutropenia (30%), pneumonia (14%), and sepsis (13%). Fatal adverse reactions occurred in 6% of patients. These included sepsis (1%), septic shock (1%), pneumonia (1%), respiratory failure (1%), and one case each of cerebral hemorrhage and sudden death.
Permanent discontinuation due to an adverse reaction occurred in 5% of patients who received INQOVI. The most frequent adverse reactions resulting in permanent discontinuation were febrile neutropenia (1%) and pneumonia (1%).
Dose interruptions due to an adverse reaction occurred in 41% of patients who received INQOVI. Adverse reactions requiring dosage interruptions in > 5% of patients who received INQOVI included neutropenia (18%), febrile neutropenia (8%), thrombocytopenia (6%), and anemia (5%).
Dose reductions due to an adverse reaction occurred in 19% of patients who received INQOVI. Adverse reactions requiring dosage reductions in > 2% of patients who received INQOVI included neutropenia (12%), anemia (3%), and thrombocytopenia (3%).
The most common adverse reactions (≥ 20%) were fatigue, constipation, hemorrhage, myalgia, mucositis, arthralgia, nausea, dyspnea, diarrhea, rash, dizziness, febrile neutropenia, edema, headache, cough, decreased appetite, upper respiratory tract infection, pneumonia, and transaminase increased. The most common Grade 3 or 4 laboratory abnormalities (≥ 50%) were leukocytes decreased, platelet count decreased, neutrophil count decreased, and hemoglobin decreased.
Table 2 summarizes the adverse reactions in the pooled safety population.
Clinically relevant adverse reactions in < 10% of patients who received INQOVI included:
- Acute febrile neutrophilic dermatosis (Sweet’s syndrome) (1%)
- Tumor lysis syndrome (0.5%)
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of intravenous decitabine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Differentiation syndrome
Respiratory, Thoracic and Mediastinal Disorders: Interstitial lung disease
Cardiac Disorders: Cardiomyopathy
5DESCRIPTION
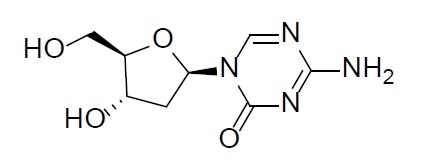
Decitabine
Decitabine is a nucleoside metabolic inhibitor. Decitabine is a white to off-white solid with the molecular formula of C
Cedazuridine
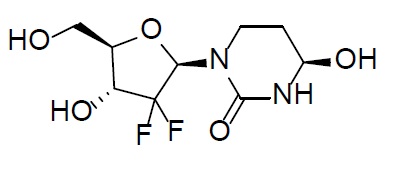
Cedazuridine is a cytidine deaminase inhibitor. Cedazuridine is a white to off-white solid with the molecular formula of C
INQOVI
INQOVI (decitabine and cedazuridine) tablets, for oral use contain 35 mg decitabine and 100 mg cedazuridine. The tablets are biconvex, oval-shaped, film-coated, red and debossed with “H35” on one side. Each film-coated tablet contains the following inactive ingredients: lactose monohydrate, hypromellose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate. The film coating material contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
6CLINICAL STUDIES
Study ASTX727-01-B
INQOVI was evaluated in Study ASTX727-01-B, an open-label, randomized, 2-cycle, 2-sequence crossover study (NCT02103478) that included 80 adult patients with MDS (International Prognostic Scoring System [IPSS] Intermediate-1, Intermediate-2, or high-risk) or CMML. Patients were randomized 1:1 to receive INQOVI (35 mg decitabine and 100 mg cedazuridine) orally in Cycle 1 and decitabine 20 mg/m
The baseline demographic and disease characteristics are shown in
Efficacy was established on the basis of complete response (CR) and the rate of conversion from transfusion dependence to transfusion independence. Efficacy results are shown in
Among the 41 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 20 (49%) became independent of RBC and platelet transfusions during any consecutive 56-day post-baseline period. Of the 39 patients who were independent of both RBC and platelet transfusions at baseline, 25 (64%) remained transfusion-independent during any consecutive 56-day post-baseline period.
Study ASTX727-02
INQOVI was evaluated in ASTX727-02, an open-label, randomized, 2-cycle, 2-sequence crossover study (NCT03306264) that included 133 adult patients with MDS or CMML, including all French-American-British (FAB) classification criteria and IPSS Intermediate-1, Intermediate-2, or high-risk prognostic scores. Patients were randomized 1:1 to receive INQOVI (35 mg decitabine and 100 mg cedazuridine) orally in Cycle 1 and decitabine 20 mg/m
The baseline demographic and disease characteristics are shown in
The primary outcome measure was comparison of the 5-day cumulative decitabine AUC between INQOVI and intravenous decitabine
Among the 57 patients who were dependent on RBC and/or platelet transfusions at baseline, 30 (53%) became independent of RBC and platelet transfusions during any 56-day post-baseline period. Of the 76 patients who were independent of both RBC and platelet transfusions at baseline, 48 (63%) remained transfusion-independent during any 56-day post-baseline period.
7REFERENCES
1. OSHA Hazardous Drugs.
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
INQOVI tablets are biconvex, oval-shaped, film-coated, red, and debossed with “H35” on one side.
The tablets are packaged in blisters and supplied as follows:
- NDC: 64842-0727-9; 5 tablets in one blister card in a child-resistant carton
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Advise patients of the risk of myelosuppression and to report any symptoms of fever, infection, anemia, or bleeding to their healthcare provider as soon as possible. Advise patients for the need for laboratory monitoring
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy
Advise females of reproductive potential to use effective contraception during treatment with INQOVI and for 6 months after the last dose
Advise males with female partners of reproductive potential to use effective contraception during treatment with INQOVI and for 3 months after the last dose
Lactation
Advise women not to breastfeed during treatment with INQOVI and for 2 weeks after the last dose
Administration
Advise patients to take INQOVI at approximately the same time each day on an empty stomach. Instruct patients to avoid eating for at least 2 hours before and 2 hours after taking INQOVI. Advise patients on what to do when a dose is missed or vomited
10PRINCIPAL DISPLAY PANEL - 35 mg/100 mg Tablet Carton
NDC 64842-0727-9
Rx Only
Rx Only
INQOVI
35 mg/100 mg per tablet
CAUTION: Hazardous Agent
One Blister card
TAIHO
