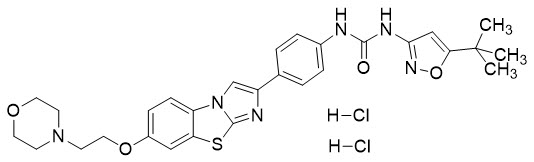
Vanflyta
What is Vanflyta (Quizartinib)?
Approved To Treat
Related Clinical Trials
Summary: This phase I/II trial studies the side effects and best dose of CPX-351 in combination with quizartinib for the treatment of acute myeloid leukemia and high risk myelodysplastic syndrome. CPX-351, composed of chemotherapy drugs daunorubicin and cytarabine, works in different ways to stop the growth of cancer cells, either by killing the cells, by stopping them from dividing, or by stopping them fr...
Summary: This phase I/II trial studies the side effects and how well cladribine, idarubicin, cytarabine, and quizartinib work in treating patients with acute myeloid leukemia or high-risk myelodysplastic syndrome that is newly diagnosed, has come back (relapsed), or does not respond to treatment (refractory). Drugs used in chemotherapy, such as cladribine, idarubicin, and cytarabine, work in different ways...
Summary: This study will compare the effects of Quizartinib versus placebo in combination with chemotherapy in participants with newly diagnosed FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) negative acute myeloid leukemia (AML).
Related Latest Advances
Brand Information
- VANFLYTA prolongs the QT interval in a dose- and concentration-related manner
- Torsades de pointes and cardiac arrest have occurred in patients receiving VANFLYTA. Do not administer VANFLYTA to patients with severe hypokalemia, severe hypomagnesemia, or long QT syndrome
- Do not initiate treatment with VANFLYTA or escalate the VANFLYTA dose if the QT interval corrected by Fridericia's formula (QTcF) is greater than 450 ms
- Monitor ECGs more frequently if concomitant use of drugs known to prolong the QT interval is required
- Reduce the VANFLYTA dose when used concomitantly with strong CYP3A inhibitors, as they may increase quizartinib exposure
- Because of the risk of QT prolongation, VANFLYTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the VANFLYTA REMS
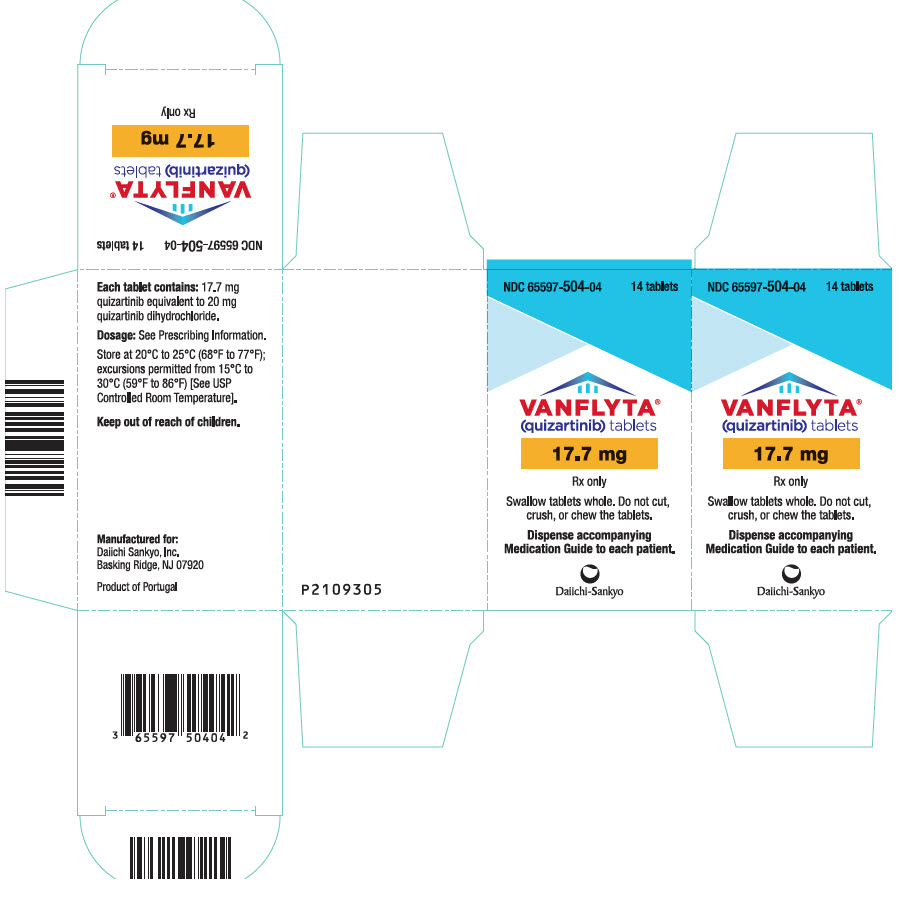
- 17.7 mg quizartinib, white, round, film-coated, debossed with "DSC511"
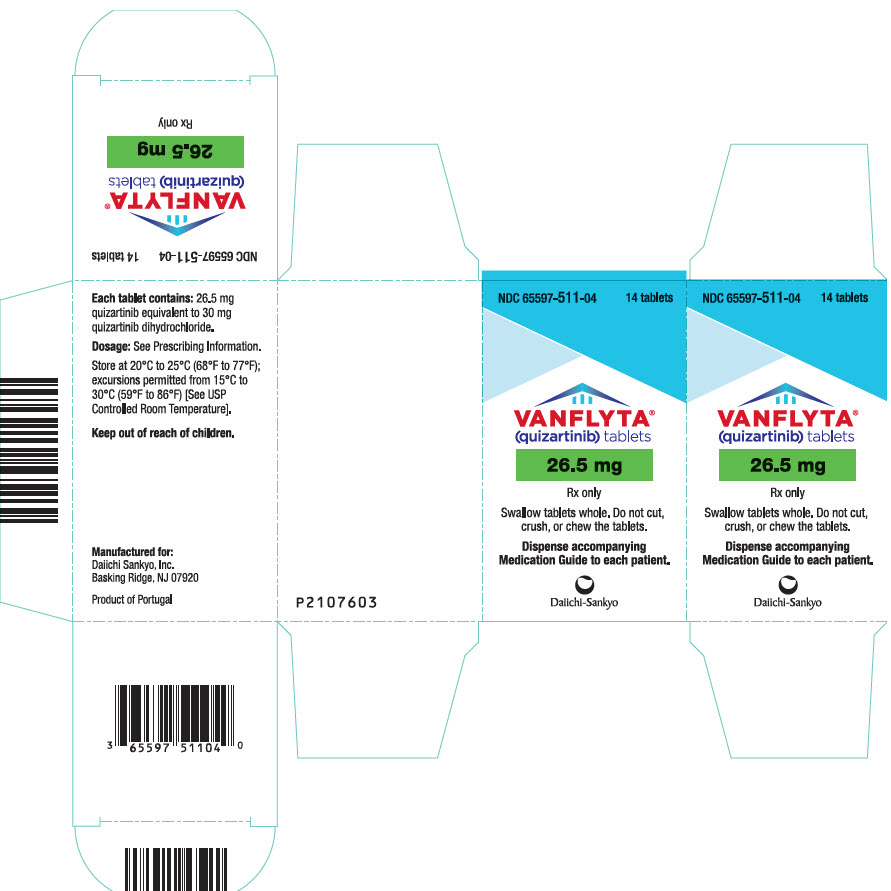
- 26.5 mg quizartinib, yellow, round, film-coated, debossed with "DSC512"
- QT Prolongation, Torsades de Pointes, and Cardiac Arrest