Lumakras
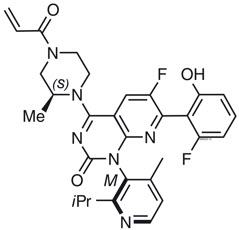
What is Lumakras (Sotorasib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase II ComboMATCH treatment trial tests how well AMG 510 (sotorasib) with or without panitumumab works in treating patients with KRAS G12C mutant solid tumors that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Sotorasib is in a class of medications called KRAS inhibitors. It works by blocking the action of the abnormal pr...
Summary: This ComboMATCH patient screening trial is the gateway to a coordinated set of clinical trials to study cancer treatment directed by genetic testing. Patients with solid tumors that have spread to nearby tissue or lymph nodes (locally advanced) or have spread to other places in the body (advanced) and have progressed on at least one line of standard systemic therapy or have no standard treatment t...
Summary: The aim of this study is to compare progression free survival (PFS) in treatment-naïve participants with KRAS p.G12C mutated metastatic colorectal cancer (mCRC) receiving sotorasib, panitumumab and FOLFIRI vs FOLFIRI with or without bevacizumab-awwb.
Related Latest Advances
Brand Information
- Hepatotoxicity
- Interstitial Lung Disease (ILD)/Pneumonitis