Generic Name
Tolterodine
Brand Names
Detrol, Tolterodine Tartarate
FDA approval date: March 25, 1998
Classification: Cholinergic Muscarinic Antagonist
Form: Tablet, Capsule
What is Detrol (Tolterodine)?
Tolterodine tartrate extended-release capsules, USP are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. Tolterodine tartrate extended-release capsules, USP are an antimuscarinic indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Detrol (tolterodine tartrate)
1DESCRIPTION
DETROL Tablets contain tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is (R)-2-[3-[bis(1-methylethyl)-amino]1-phenylpropyl]-4-methylphenol [R-(R*,R*)]-2,3dihydroxybutanedioate (1:1) (salt). The empirical formula of tolterodine tartrate is C
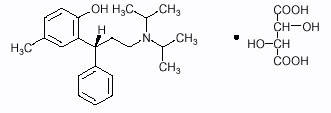
Tolterodine tartrate is a white, crystalline powder. The pKa value is 9.87 and the solubility in water is 12 mg/mL. It is soluble in methanol, slightly soluble in ethanol, and practically insoluble in toluene. The partition coefficient (Log D) between n-octanol and water is 1.83 at pH 7.3.
DETROL Tablets for oral administration contain 1 or 2 mg of tolterodine tartrate. The inactive ingredients are colloidal anhydrous silica, calcium hydrogen phosphate dihydrate, cellulose microcrystalline, hypromellose, magnesium stearate, sodium starch glycolate (pH 3.0 to 5.0), stearic acid, and titanium dioxide.
2CLINICAL PHARMACOLOGY
Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors.
After oral administration, tolterodine is metabolized in the liver, resulting in the formation of the 5-hydroxymethyl derivative, a major pharmacologically active metabolite. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and the 5-hydroxymethyl metabolite exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels.
Tolterodine has a pronounced effect on bladder function. Effects on urodynamic parameters before and 1 and 5 hours after a single 6.4 mg dose of tolterodine immediate release were determined in healthy volunteers. The main effects of tolterodine at 1 and 5 hours were an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure. These findings are consistent with an antimuscarinic action on the lower urinary tract.
2.1Cardiac Electrophysiology
The effect of 2 mg BID and 4 mg BID of tolterodine immediate release (IR) on the QT interval was evaluated in a 4-way crossover, double-blind, placebo- and active-controlled (moxifloxacin 400 mg QD) study in healthy male (N=25) and female (N=23) volunteers aged 18–55 years. Study subjects [approximately equal representation of CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs)] completed sequential 4-day periods of dosing with moxifloxacin 400 mg QD, tolterodine 2 mg BID, tolterodine 4 mg BID, and placebo. The 4 mg BID dose of tolterodine IR (two times the highest recommended dose) was chosen because this dose results in tolterodine exposure similar to that observed upon coadministration of tolterodine 2 mg BID with potent CYP3A4 inhibitors in patients who are CYP2D6 poor metabolizers (see
Table 2 summarizes the mean change from baseline to steady state in corrected QT interval (QTc) relative to placebo at the time of peak tolterodine (1 hour) and moxifloxacin (2 hour) concentrations. Both Fridericia’s (QTcF) and a population-specific (QTcP) method were used to correct QT interval for heart rate. No single QT correction method is known to be more valid than others. QT interval was measured manually and by machine, and data from both are presented. The mean increase of heart rate associated with a 4 mg/day dose of tolterodine in this study was 2.0 beats/minute and 6.3 beats/minute with 8 mg/day tolterodine. The change in heart rate with moxifloxacin was 0.5 beats/minute.
The reason for the difference between machine and manual read of QT interval is unclear.
The QT effect of tolterodine immediate release tablets appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day. The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped.
Tolterodine’s effect on QT interval was found to correlate with plasma concentration of tolterodine. There appeared to be a greater QTc interval increase in CYP2D6 poor metabolizers than in CYP2D6 extensive metabolizers after tolterodine treatment in this study.
This study was not designed to make direct statistical comparisons between drugs or dose levels. There has been no association of Torsade de Pointes in the international post-marketing experience with DETROL or DETROL LA (see
3CLINICAL STUDIES
DETROL Tablets were evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in four randomized, double-blind, placebo-controlled, 12-week studies. A total of 853 patients received DETROL 2 mg twice daily and 685 patients received placebo. The majority of patients were Caucasian (95%) and female (78%), with a mean age of 60 years (range, 19 to 93 years). At study entry, nearly all patients perceived they had urgency and most patients had increased frequency of micturitions and urge incontinence. These characteristics were well balanced across treatment groups for the studies.
The efficacy endpoints for study 007 (see Table 3) included the change from baseline for:
- Number of incontinence episodes per week
- Number of micturitions per 24 hours (averaged over 7 days)
- Volume of urine voided per micturition (averaged over 2 days)
The efficacy endpoints for studies 008, 009, and 010 (see Table 4) were identical to the above endpoints with the exception that the number of incontinence episodes was per 24 hours (averaged over 7 days).
4INDICATIONS AND USAGE
DETROL Tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
5CONTRAINDICATIONS
DETROL Tablets are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. DETROL is also contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like DETROL, are metabolized to 5-hydroxymethyl tolterodine.
6WARNINGS
Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of DETROL. In the event of difficulty in breathing, upper airway obstruction, or fall in blood pressure, DETROL should be discontinued and appropriate therapy promptly provided.
7ADVERSE REACTIONS
The Phase 2 and 3 clinical trial program for DETROL Tablets included 3071 patients who were treated with DETROL (N=2133) or placebo (N=938). The patients were treated with 1, 2, 4, or 8 mg/day for up to 12 months. No differences in the safety profile of tolterodine were identified based on age, gender, race, or metabolism.
The data described below reflect exposure to DETROL 2 mg bid in 986 patients and to placebo in 683 patients exposed for 12 weeks in five Phase 3, controlled clinical studies. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and approximating rates.
Sixty-six percent of patients receiving DETROL 2 mg bid reported adverse events versus 56% of placebo patients. The most common adverse events reported by patients receiving DETROL were dry mouth, headache, constipation, vertigo/dizziness, and abdominal pain. Dry mouth, constipation, abnormal vision (accommodation abnormalities), urinary retention, and xerophthalmia are expected side effects of antimuscarinic agents.
Dry mouth was the most frequently reported adverse event for patients treated with DETROL 2 mg bid in the Phase 3 clinical studies, occurring in 34.8% of patients treated with DETROL and 9.8% of placebo-treated patients. One percent of patients treated with DETROL discontinued treatment due to dry mouth.
The frequency of discontinuation due to adverse events was highest during the first 4 weeks of treatment. Seven percent of patients treated with DETROL 2 mg bid discontinued treatment due to adverse events versus 6% of placebo patients. The most common adverse events leading to discontinuation of DETROL were dizziness and headache.
Three percent of patients treated with DETROL 2 mg bid reported a serious adverse event versus 4% of placebo patients. Significant ECG changes in QT and QTc have not been demonstrated in clinical-study patients treated with DETROL 2 mg bid. Table 5 lists the adverse events reported in 1% or more of the patients treated with DETROL 2 mg bid in the 12-week studies. The adverse events are reported regardless of causality.
7.1Post-marketing Surveillance
The following events have been reported in association with tolterodine use in worldwide post-marketing experience:
Reports of aggravation of symptoms of dementia (e.g., confusion, disorientation, delusion) have been reported after tolterodine therapy was initiated in patients taking cholinesterase inhibitors for the treatment of dementia.
Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of tolterodine in their causation cannot be reliably determined.
8OVERDOSAGE
A 27-month-old child who ingested 5 to 7 DETROL Tablets 2 mg was treated with a suspension of activated charcoal and was hospitalized overnight with symptoms of dry mouth. The child fully recovered.
8.1Management of Overdosage
Overdosage with DETROL can potentially result in severe central anticholinergic effects and should be treated accordingly.
ECG monitoring is recommended in the event of overdosage. In dogs, changes in the QT interval (slight prolongation of 10% to 20%) were observed at a suprapharmacologic dose of 4.5 mg/kg, which is about 68 times higher than the recommended human dose. In clinical trials of normal volunteers and patients, QT interval prolongation was observed with tolterodine immediate release at doses up to 8 mg (4 mg bid) and higher doses were not evaluated (see
9DOSAGE AND ADMINISTRATION
The initial recommended dose of DETROL Tablets is 2 mg twice daily. The dose may be lowered to 1 mg twice daily based on individual response and tolerability. For patients with significantly reduced hepatic or renal function or who are currently taking drugs that are potent inhibitors of CYP3A4, the recommended dose of DETROL is 1 mg twice daily (see
10HOW SUPPLIED
DETROL Tablets 1 mg (white, round, biconvex, film-coated tablets engraved with arcs above and below the letters “TO”) and DETROL Tablets 2 mg (white, round, biconvex, film-coated tablets engraved with arcs above and below the letters “DT”) are supplied as follows:
Bottles of 60
Store at 25ºC (77ºF); excursions permitted to 15–30ºC (59–86ºF) [see USP Controlled Room Temperature] (DTL).
Distributed by:
Made in Italy
© 2023 Viatris Inc.
DETROL is a registered trademark of Viatris Specialty LLC, a Viatris Company.
The brands listed are trademarks of their respective owners.
UPJ:DTRLT:R1
Revised: 2/2023
11PATIENT INFORMATION
DETROL(DE-trol)
Read the Patient Information that comes with DETROL before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or your treatment. Only your doctor can determine if treatment with DETROL is right for you.
What is DETROL?
DETROL is a prescription medicine for
- Urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- Urgency: a strong need to urinate right away
- Frequency: urinating often
DETROL LA (tolterodine tartrate extended release capsules) did not help the symptoms of overactive bladder when studied in children.
What is overactive bladder?
Overactive bladder happens when you cannot control your bladder muscle. When the muscle contracts too often or cannot be controlled, you get symptoms of overactive bladder, which are leakage of urine (urge urinary incontinence), needing to urinate right away (urgency), and needing to urinate often (frequency).
Who should not take DETROL?
Do not take DETROL if you:
- Are not able to empty your bladder (urinary retention)
- Have delayed or slow emptying of your stomach (gastric retention)
- Have an eye problem called “uncontrolled narrow-angle glaucoma”
- Are allergic to DETROL or to any of its ingredients. See the end of this leaflet for a complete list of ingredients
- Are allergic to TOVIAZ which contains fesoterodine.
What should I tell my doctor before starting DETROL?
Before starting DETROL, tell your doctor about all of your medical and other conditions that may affect the use of DETROL, including:
- Stomach or intestinal problems or problems with constipation
- Problems emptying your bladder or if you have a weak urine stream
- Treatment for an eye problem called narrow-angle glaucoma
- Liver problems
- Kidney problems
- A condition called myasthenia gravis
- If you or any family members have a rare heart condition called QT prolongation (long QT syndrome)
- If you are pregnant or trying to become pregnant. It is not known if DETROL could harm your unborn baby.
- If you are breastfeeding.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Other medicines can affect how your body handles DETROL. Your doctor may use a lower dose of DETROL if you are taking:
- Certain medicines for fungus or yeast infections
- Certain medicines for bacterial infections
- Sandimmune
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them with you to show your doctor or pharmacist each time you get a new medicine.
How should I take DETROL?
- Take DETROL exactly as your doctor tells you to take it.
- Your doctor will tell you how many DETROL Tablets to take and when to take them.
- Do not change your dose unless told to do so by your doctor.
- You can take DETROL with or without food.
- Take DETROL at the same times each day.
- If you miss a dose of DETROL, just take your next regular dose at your next regular time. Do not try to make up for your missed dose.
- If you take too much DETROL, call your doctor, or go to the hospital emergency room right away.
What should I avoid while taking DETROL?
Medicines like
What are possible side effects of DETROL?
DETROL may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking DETROL and get emergency medical help right away.
The most common side effects with DETROL are:
- Dry mouth
- Dizziness
- Headache
- Stomach pain
- Constipation
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all the side effects with DETROL. For a complete list, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How do I store DETROL?
- Store DETROL at room temperature (59 to 86° F).
- Keep it in a dry place.
Keep DETROL and all medicines out of the reach of children.
General Information about DETROL
Medicines are sometimes prescribed for conditions that are not mentioned in the patient information leaflet. Only use DETROL the way your doctor tells you. Do not give DETROL to other people even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about DETROL. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about DETROL that is written for health professionals.
For more information, call Viatris at 1-877-446-3679 (1-877-4-INFO-RX).
What are the ingredients in DETROL? tolterodine tartrate
Inactive ingredients: colloidal anhydrous silica, calcium hydrogen phosphate dihydrate, cellulose microcrystalline, hypromellose, magnesium stearate, sodium starch glycolate (pH 3.0 to 5.0), stearic acid, and titanium dioxide.
Inactive ingredients: colloidal anhydrous silica, calcium hydrogen phosphate dihydrate, cellulose microcrystalline, hypromellose, magnesium stearate, sodium starch glycolate (pH 3.0 to 5.0), stearic acid, and titanium dioxide.
Distributed by:
Made in Italy
© 2023 Viatris Inc.
DETROL is a registered trademark of Viatris Specialty LLC, a Viatris Company.
The brands listed are trademarks of their respective owners.
UPJ:PL:DTRLT:R1
Revised: 2/2023
12PRINCIPAL DISPLAY PANEL – 1 mg
NDC 58151-098-91
Detrol®
60 Tablets
Rx only
VIATRIS™
Store at 25°C (77°F); excursionsDispense in tight containers (USP).
DOSAGE AND USE:
See accompanying
prescribing information.
Each tablet contains
1 mg tolterodine tartrate.
DOSAGE AND USE:
See accompanying
prescribing information.
Each tablet contains
1 mg tolterodine tartrate.
Distributed by:
Made in Italy
© 2023 Viatris Inc.
RUPJ098D
PAA208141

13PRINCIPAL DISPLAY PANEL – 2 mg
NDC 58151-099-91
Detrol®
60 Tablets
Rx only
VIATRIS™
Store at 25°C (77°F); excursionsDispense in tight containers (USP).
DOSAGE AND USE:
See accompanying
prescribing information.
Each tablet contains
2 mg tolterodine tartrate.
DOSAGE AND USE:
See accompanying
prescribing information.
Each tablet contains
2 mg tolterodine tartrate.
Distributed by:
Made in Italy
© 2023 Viatris Inc.
RUPJ099D
PAA208142
