Brand Name
Dhivy
Generic Name
Carbidopa Levodopa
View Brand Information FDA approval date: February 10, 2022
Classification: Aromatic Amino Acid
Form: Tablet
What is Dhivy (Carbidopa Levodopa)?
DHIVY is indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication or manganese intoxication. DHIVY is a combination of carbidopa and levodopa indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Gait Pattern Analysis in Neurological Disease
Summary: The purpose of this study is to investigate whether speed-dependent measures of gait can be identified in patients with neurological conditions that affect gait, particularly in subjects with parkinsonian disorders.
Dopaminergic Therapy for Inflammation-Related Anhedonia in Depression - 2
Summary: The purpose of this 8-week, double-blind, placebo-controlled, study is to explore new treatment options for people with depression who have high inflammation and anhedonia. Seventy male and female participants with depression, between 25-55 years of age, with higher levels of inflammation and anhedonia will be randomized to receive L-DOPA or matched placebo over 8 weeks. Participants will complete...
Related Latest Advances
Brand Information
Dhivy (Carbidopa Levodopa)
1INDICATIONS AND USAGE
DHIVY is indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.
2DOSAGE FORMS AND STRENGTHS
DHIVY tablets are white to off-white tablets containing 25 mg of carbidopa and 100 mg of levodopa. Each DHIVY tablet has 3 functional scores with each segment containing 6.25 mg of carbidopa and 25 mg of levodopa.
3CONTRAINDICATIONS
DHIVY is contraindicated in patients
- Currently taking a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine, linezolid, and tranylcypromine) or have recently (within 2 weeks) taken a nonselective MAO inhibitor. Hypertension can occur if these drugs are used concurrently
- With known hypersensitivity to any component of DHIVY
4ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Falling Asleep During Activities of Daily Living and Somnolence
- Withdrawal-Emergent Hyperpyrexia and Confusion
- Cardiovascular Ischemic Events
- Hallucinations/Psychotic-Like Behavior
- Impulse Control/Compulsive Behaviors
- Dyskinesia
- Peptic Ulcer Disease
- Glaucoma
- Depression//Suicidality
The most common adverse reactions reported with carbidopa/levodopa tablets have included dyskinesias, such as choreiform, dystonic, and other involuntary movements, and nausea.
The following other adverse reactions have been reported with carbidopa/levodopa tablets:
Body as a Whole
Chest pain, asthenia.
Chest pain, asthenia.
Cardiovascular
Cardiac irregularities, hypotension, orthostatic effects including orthostatic hypotension, hypertension, syncope, phlebitis, palpitation.
Cardiac irregularities, hypotension, orthostatic effects including orthostatic hypotension, hypertension, syncope, phlebitis, palpitation.
Gastrointestinal
Dark saliva, gastrointestinal bleeding, development of duodenal ulcer, anorexia, vomiting, diarrhea, constipation, dyspepsia, dry mouth, taste alterations.
Dark saliva, gastrointestinal bleeding, development of duodenal ulcer, anorexia, vomiting, diarrhea, constipation, dyspepsia, dry mouth, taste alterations.
Hematologic
Agranulocytosis, hemolytic and non-hemolytic anemia, thrombocytopenia, leukopenia.
Agranulocytosis, hemolytic and non-hemolytic anemia, thrombocytopenia, leukopenia.
Hypersensitivity
Angioedema, urticaria, pruritus, Henoch-Schönlein purpura, bullous lesions (including pemphigus-like reactions).
Angioedema, urticaria, pruritus, Henoch-Schönlein purpura, bullous lesions (including pemphigus-like reactions).
Musculoskeletal
Back pain, shoulder pain, muscle cramps.
Back pain, shoulder pain, muscle cramps.
Nervous System/Psychiatric
Psychotic episodes including delusions, hallucinations, and paranoid ideation, bradykinetic episodes (“on-off” phenomenon), confusion, agitation, dizziness, somnolence, dream abnormalities including nightmares, insomnia, paresthesia, headache, depression with or without development of suicidal tendencies, dementia, pathological gambling, increased libido including hypersexuality, impulse control symptoms. Convulsions also have occurred; however, a causal relationship with DHIVY has not been established.
Psychotic episodes including delusions, hallucinations, and paranoid ideation, bradykinetic episodes (“on-off” phenomenon), confusion, agitation, dizziness, somnolence, dream abnormalities including nightmares, insomnia, paresthesia, headache, depression with or without development of suicidal tendencies, dementia, pathological gambling, increased libido including hypersexuality, impulse control symptoms. Convulsions also have occurred; however, a causal relationship with DHIVY has not been established.
Respiratory
Dyspnea, upper respiratory infection.
Dyspnea, upper respiratory infection.
Skin
Rash, increased sweating, alopecia, dark sweat.
Rash, increased sweating, alopecia, dark sweat.
Urogenital
Urinary tract infection, urinary frequency, dark urine.
Urinary tract infection, urinary frequency, dark urine.
Laboratory Tests
Decreased hemoglobin and hematocrit; abnormalities in alkaline phosphatase, SGOT (AST), SGPT (ALT), LDH, bilirubin, BUN, Coombs test; elevated serum glucose; white blood cells, bacteria, and blood in the urine.
Decreased hemoglobin and hematocrit; abnormalities in alkaline phosphatase, SGOT (AST), SGPT (ALT), LDH, bilirubin, BUN, Coombs test; elevated serum glucose; white blood cells, bacteria, and blood in the urine.
Other adverse reactions that have been reported with levodopa alone and with various carbidopa levodopa formulations, and may occur with DHIVY are:
Body as a Whole
Abdominal pain and distress, fatigue.
Abdominal pain and distress, fatigue.
Cardiovascular
Myocardial infarction.
Myocardial infarction.
Gastrointestinal
Gastrointestinal pain, dysphagia, sialorrhea, flatulence, bruxism, burning sensation of the tongue, heartburn, hiccups.
Gastrointestinal pain, dysphagia, sialorrhea, flatulence, bruxism, burning sensation of the tongue, heartburn, hiccups.
Metabolic
Edema, weight gain, weight loss.
Edema, weight gain, weight loss.
Musculoskeletal
Leg pain.
Leg pain.
Nervous System/Psychiatric
Ataxia, extrapyramidal disorder, falling, anxiety, gait abnormalities, nervousness, decreased mental acuity, memory impairment, disorientation, euphoria, blepharospasm (which may be taken as an early sign of excess dosage; consideration of dosage reduction may be made at this time), trismus, increased tremor, numbness, muscle twitching, activation of latent Horner’s syndrome, peripheral neuropathy.
Ataxia, extrapyramidal disorder, falling, anxiety, gait abnormalities, nervousness, decreased mental acuity, memory impairment, disorientation, euphoria, blepharospasm (which may be taken as an early sign of excess dosage; consideration of dosage reduction may be made at this time), trismus, increased tremor, numbness, muscle twitching, activation of latent Horner’s syndrome, peripheral neuropathy.
Respiratory
Pharyngeal pain, cough.
Pharyngeal pain, cough.
Skin
Malignant melanoma, flushing.
Malignant melanoma, flushing.
Special Senses
Oculogyric crises, diplopia, blurred vision, dilated pupils.
Oculogyric crises, diplopia, blurred vision, dilated pupils.
Urogenital
Urinary retention, urinary incontinence, priapism.
Urinary retention, urinary incontinence, priapism.
Miscellaneous
Bizarre breathing patterns, faintness, hoarseness, malaise, hot flashes, sense of stimulation.
Bizarre breathing patterns, faintness, hoarseness, malaise, hot flashes, sense of stimulation.
5OVERDOSAGE
Based on the limited available information, the acute symptoms of levodopa/dopa decarboxylase inhibitor overdosage can be expected to arise from dopaminergic overstimulation. Doses of a few grams may result in CNS disturbances, with an increasing likelihood of cardiovascular disturbance (e.g., hypotension, tachycardia) and more severe psychiatric problems at higher doses. An isolated report of rhabdomyolysis and another of transient renal insufficiency suggest that levodopa overdosage may give rise to systemic complications, secondary to dopaminergic overstimulation.
Monitor patients and provide supportive care. Patients should receive electrocardiographic monitoring for the development of arrhythmias; if needed, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs, increasing the risk of drug interactions (especially catechol-structured drugs) should be taken into consideration.
6DESCRIPTION
DHIVY
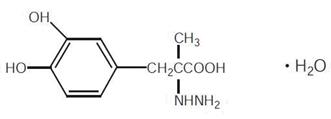
Carbidopa is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.3. It is designated chemically as (–)-L-α-hydrazino-α-methyl-β-(3,4-dihydroxy-benzene) propanoic acid monohydrate. It has a pKa of 2.3. Its molecular formula is C
Tablet content is expressed in terms of anhydrous carbidopa, which has a molecular weight of 226.3.
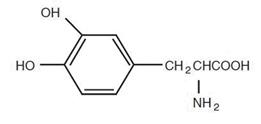
Levodopa is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.2. It is designated chemically as (–)-L-α-amino-β-(3,4-dihydroxybenzene) propanoic acid. It has a pKa of 2.32. Its molecular formula is C
DHIVY is supplied as tablets for oral administration containing 25 mg of carbidopa and 100 mg of levodopa. The inactive ingredients are magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
7CLINICAL STUDIES
The efficacy of DHIVY is based upon bioavailability studies comparing DHIVY to an immediate-release tablet containing 25 mg of carbidopa and 100 mg of levodopa
8PATIENT COUNSELING INFORMATION
Dosing Instructions
- It is important that DHIVY be taken at regular intervals according to the schedule outlined by their physician. Inform the patient not to change the prescribed dosage regimen and not to add any additional antiparkinson medications, including other carbidopa-levodopa preparations, without first consulting their physician. Advise patients to call their healthcare provider before stopping DHIVY. Discontinue DHIVY slowly. Tell patients to call their healthcare provider if they develop withdrawal symptoms such as fever and confusion
- Advise patients to swallow DHIVY with or without food. If the patient has difficulty swallowing the tablet due to its size, inform the patient that the tablet can be broken at the score lines
- Advise the patient that occasionally, dark color (red, brown, or black) may appear in saliva, urine, or sweat after ingestion of DHIVY. Although the color appears to be clinically insignificant, garments may become discolored.
- Advise the patient that a change in diet to foods that are high in protein or taking iron salts or multivitamins with iron may delay the absorption of levodopa and may reduce the amount taken up in the circulation. Excessive acidity also delays stomach emptying, thus delaying the absorption of levodopa.
Falling Asleep
Advise patients that certain side effects such as sleepiness and dizziness that have been reported with DHIVY may affect some patients’ ability to drive and operate machinery safely
Hallucinations and Psychosis
Inform patients that hallucinations can occur with levodopa products
Impulse Control Disorder
Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson’s disease
Dyskinesia
Instruct patients to notify their healthcare provider if abnormal involuntary movements appear or get worse during treatment with DHIVY
Pregnancy and Breastfeeding
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during DHIVY therapy
Advise female patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant
Distributed by:
L-0344 Rev. 0423-02
INSTRUCTIONS FOR USE
DHIVY (DHI-VEE)
(carbidopa and levodopa)
tablets, for oral use
This Instructions for Use contains information on how to take DHIVY.
Important Information You Need to Know Before Taking DHIVY
- Take DHIVY exactly as your healthcare provider tells you to take it. Your healthcare provider may increase your dose every day or every other day as needed until you reach the recommended dosage.
- Take DHIVY by mouth, with or without food.
- 1 whole DHIVY tablet contains 25 mg of carbidopa and 100 mg of levodopa.
- 1 DHIVY tablet has 3 grooves (score lines) that can be split into 4 segments so that you can take your prescribed dose. (See the DHIVY tablet image below.)
- If you have trouble swallowing the DHIVY tablet, you can break the tablet at the groove (score lines). Then take the number of segments that equal your prescribed dose. Each segment of 1 DHIVY tablet contains 6.25 mg of carbidopa and 25 mg of levodopa. (See Table 1 below to help determine how many tablet segments to take.)
- Do not change your dose or take any additional antiparkinson medicines (including medicines that contain carbidopa and levodopa) without first talking to your healthcare provider. If you are not sure if you take these medicines, ask your healthcare provider.
- Call your healthcare provider before you stop taking DHIVY. Call your healthcare provider if you develop withdrawal symptoms such as fever and confusion.
- Changing your diet to highly acidic foods or foods that are high in protein or taking iron salts or multivitamins with iron may affect how DHIVY works. If you are not sure if you are on any of these diets or taking any of these medicines, ask your healthcare provider.
DHIVY Tablet

Dhivy tablet has four segments. Each segment contains 6.25 mg of carbidopa and 25 mg of levodopa. The top layer of the tablet (above the dotted line) contains the carbidopa and levodopa. The bottom layer of the tablet (below the dotted line) contains the inactive ingredients.
Table 1: DHIVY Segment Description
How to take 3 Segments (18.75 mg of carbidopa and 75 mg of levodopa)
- Hold the DHIVY tablet with 1 hand on each side.
- Carefully grasp 1 segment on either side and break the tablet at the groove to get 3 segments containing 18.75 mg of carbidopa and 75 mg of levodopa and 1 segment containing 6.25 mg of carbidopa and 25 mg of levodopa.
- Put the remaining 1 segment back into the container and close tightly.
How to take 2 Segments (12.5 mg of carbidopa and 50 mg of levodopa)
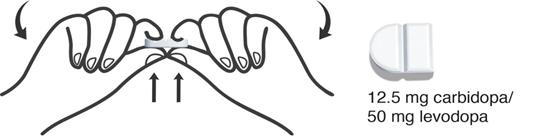
- Hold the DHIVY tablet with 1 hand on each side.
- Carefully grasp 2 segments and break the tablet in half at the groove in the middle to get 2 segments containing 12.5 mg of carbidopa and 50 mg of levodopa each.
- Put the remaining 2 segments back into the container and close tightly.
How to take 1 Segment (6.25 mg of carbidopa and 25 mg of levodopa)
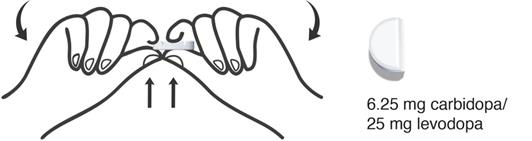
- Hold the DHIVY tablet with 1 hand on each side.
- Carefully grasp 1 segment on either side and break the tablet at the groove to get 1 segment containing 6.25 mg of carbidopa and 25 mg of levodopa and 3 segments containing 18.75 mg of carbidopa and 75 mg of levodopa.
- Put the remaining 3 segments back into the container and close tightly.
Storing DHIVY
- Store DHIVY at room temperature between 68°F to 77°F (20°C to 25°C).
- Store DHIVY in a tightly closed container to protect it from light and moisture.
- Keep DHIVY and all medicines out of the reach of children.
To report side effects, call Avion Pharmaceuticals at 888-612-8466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Distributed by:
Avion Pharmaceuticals, LLC
Alpharetta, GA, 30005 USA
1-888-612-8466
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 06/2022
9PACKAGE LABEL.PRINCIPAL DISPLAY PANEL