Generic Name
Fenofibrate
Brand Names
Lipofen, Tricor
FDA approval date: April 09, 2002
Classification: Peroxisome Proliferator Receptor alpha Agonist
Form: Tablet, Capsule
What is Lipofen (Fenofibrate)?
TRICOR is a peroxisome proliferator-activated receptor alpha agonist indicated as an adjunct to diet: To reduce elevated LDL-C, Total-C, TG and Apo B, and to increase HDL-C in adult patients with primary hypercholesterolemia or mixed dyslipidemia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Lipofen (fenofibrate)
1INDICATIONS AND USAGE
LIPOFEN is indicated as adjunctive therapy to diet:
- to reduce triglyceride (TG) levels in adults with severe hypertriglyceridemia (TG greater than or equal to 500 mg/dL).
- to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia when use of recommended LDL-C lowering therapy is not possible.
2DOSAGE FORMS AND STRENGTHS
- 50 mg: Size 3 white opaque gelatin capsule imprinted "G 246" and "50" in black ink.
- 150 mg: Size 1 white opaque gelatin capsule imprinted "G 248" and "150" in green ink.
3CONTRAINDICATIONS
LIPOFEN is contraindicated in patients with:
- Severe renal impairment, including those with end-stage renal disease (ESRD) and those receiving dialysis
- Active liver disease, including those with unexplained persistent liver function abnormalities
- Pre-existing gallbladder disease
- Hypersensitivity to fenofibrate, fenofibric acid, or any of the excipients in LIPOFEN. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with fenofibrate
4ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Mortality and coronary heart disease morbidity
- Hepatoxicity
- Myopathy and Rhabdomyolysis
- Increases in Serum Creatinine
- Cholelithiasis
- Increased Bleeding Risk with Coumarin Anticoagulants
- Pancreatitis
- Hematologic Changes
- Hypersensitivity reactions
- Venothromboembolic disease
- Paradoxical Decreases in HDL Cholesterol Levels
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of LIPOFEN has been established in adults with hypertriglyceridemia or primary hyperlipidemia based on adequate and well-controlled trials of other formulations of fenofibrate, referenced below as "fenofibrate"
Adverse reactions reported by 2% or more of patients treated with fenofibrate (and greater than placebo) during the double-blind, placebo-controlled trials are listed in Table 1 below. Adverse reactions led to discontinuation of treatment in 5% of patients treated with fenofibrate and in 3% treated with placebo. Increases in liver function tests were the most frequent events, causing discontinuation of fenofibrate treatment in 1.6% of patients in double-blind trials.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fenofibrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Blood: Anemia, white blood cell decreases
Gastrointestinal: Pancreatitis
General: Asthenia
Hepatobiliary: Increased total bilirubin, hepatitis, cirrhosis
Immune System: Anaphylaxis, angioedema
Lipid Disorders: Severely depressed HDL-cholesterol levels
Musculoskeletal: Myalgia, muscle spasms, rhabdomyolysis, arthralgia
Renal and Urinary: Acute renal failure
Respiratory: Interstitial lung disease
Skin and Subcutaneous Tissue: Photosensitivity reactions, days to months after initiation. This may occur in patients who report a prior photosensitivity reaction to ketoprofen.
5DRUG INTERACTIONS
Table 2 presents clinically important drug interactions with LIPOFEN.
6OVERDOSAGE
In the event of an overdose of LIPOFEN, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
There is no specific treatment for overdose with LIPOFEN. General supportive care of the patient is indicated, including monitoring of vital signs and observation of clinical status, should an overdose occur. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage, usual precautions should be observed to maintain the airway. Because fenofibric acid is highly bound to plasma proteins, hemodialysis should not be considered.
7DESCRIPTION
LIPOFEN
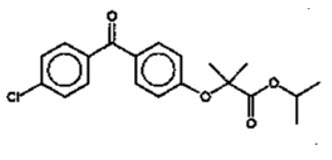
The empirical formula is C
LIPOFEN (fenofibrate capsules, USP) meets USP Dissolution Test 2.
Inactive Ingredients: Each hard gelatin capsule contains Gelucire 44/14 (lauroyl macrogol glyceride type 1500), polyethylene glycol 20,000, polyethylene glycol 8000, hydroxypropylcellulose, sodium starch glycolate, gelatin, titanium dioxide, shellac, propylene glycol, may also contain black iron oxide, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, D&C Yellow #10.
8HOW SUPPLIED/STORAGE AND HANDLING
LIPOFEN
9PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
NDC 62559-305-90
Lipofen
50 mg
Rx Only
ani
90 Capsules

10PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
NDC 62559-306-90
Lipofen
150 mg
Rx Only
ani
90 Capsules
