Generic Name
Zonisamide
Brand Names
Zonegran, Zonisade
FDA approval date: March 27, 2000
Classification: Anti-epileptic Agent
Form: Suspension, Capsule
What is Zonegran (Zonisamide)?
Zonisamide capsules are indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Zonegran (Zonisamide)
1DESCRIPTION
ZONEGRAN
The chemical structure is:
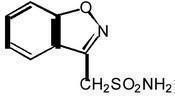
ZONEGRAN is supplied for oral administration as capsules containing 25 mg or 100 mg zonisamide.
Each 25 mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide.
Each 100 mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, titanium dioxide, FD&C Red No. 40 and FD&C Yellow No. 6.
2INDICATIONS AND USAGE
ZONEGRAN is indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy.
3CONTRAINDICATIONS
ZONEGRAN is contraindicated in patients who have demonstrated hypersensitivity to sulfonamides or zonisamide.
4WARNINGS
Potentially Fatal Reactions to Sulfonamides: Fatalities have occurred, although rarely, as a result of severe reactions to sulfonamides (zonisamide is a sulfonamide) including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Such reactions may occur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue zonisamide immediately. Specific experience with sulfonamide-type adverse reaction to zonisamide is described below.
4.1Oligohidrosis and Hyperthermia in Pediatric Patients:
Oligohidrosis, sometimes resulting in heat stroke and hospitalization, is seen in association with zonisamide in pediatric patients.
During the pre-approval development program in Japan, one case of oligohidrosis was reported in 403 pediatric patients, an incidence of 1 case per 285 patient-years of exposure. While there were no cases reported in the US or European development programs, fewer than 100 pediatric patients participated in these trials.
In the first 11 years of marketing in Japan, 38 cases were reported, an estimated reporting rate of about 1 case per 10,000 patient-years of exposure. In the first year of marketing in the US, 2 cases were reported, an estimated reporting rate of about 12 cases per 10,000 patient-years of exposure. These rates are underestimates of the true incidence because of under-reporting. There has also been one report of heat stroke in an 18-year-old patient in the US.
Decreased sweating and an elevation in body temperature above normal characterized these cases. Many cases were reported after exposure to elevated environmental temperatures. Heat stroke, requiring hospitalization, was diagnosed in some cases. There have been no reported deaths.
Pediatric patients appear to be at an increased risk for zonisamide-associated oligohidrosis and hyperthermia. Patients, especially pediatric patients, treated with ZONEGRAN should be monitored closely for evidence of decreased sweating and increased body temperature, especially in warm or hot weather. Caution should be used when zonisamide is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include, but are not limited to, carbonic anhydrase inhibitors and drugs with anticholinergic activity.
The practitioner should be aware that the safety and effectiveness of zonisamide in pediatric patients have not been established, and that zonisamide is not approved for use in pediatric patients.
4.2Acute Myopia and Secondary Angle Closure Glaucoma:
Acute myopia and secondary angle closure glaucoma have been reported in patients receiving ZONEGRAN. Elevated intraocular pressure can lead to serious sequelae, including permanent vision loss, if left untreated.
4.3Suicidal Behavior and Ideation:
Antiepileptic drugs (AEDs), including ZONEGRAN, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing ZONEGRAN or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers (see
4.4Metabolic Acidosis:
Zonisamide causes hyperchloremic, non-anion gap, metabolic acidosis (i.e., decreased serum bicarbonate below the normal reference range in the absence of chronic respiratory alkalosis) (see
Conditions or therapies that predispose to acidosis (such as renal disease, severe respiratory disorders, status epilepticus, diarrhea, ketogenic diet, or specific drugs) may be additive to the bicarbonate lowering effects of zonisamide.
Some manifestations of acute or chronic metabolic acidosis include hyperventilation, nonspecific symptoms such as fatigue and anorexia, or more severe sequelae including cardiac arrhythmias or stupor. Chronic, untreated, metabolic acidosis may increase the risk for nephrolithiasis or nephrocalcinosis. Nephrolithiasis has been observed in the clinical development program in 4% of adults treated with ZONEGRAN, has also been detected by renal ultrasound in 8% of pediatric treated patients who had at least one ultrasound prospectively collected, and was reported as an adverse event in 3% (4/133) of pediatric patients (see
Chronic, untreated metabolic acidosis may result in osteomalacia (referred to as rickets in pediatric patients) and/or osteoporosis with an increased risk for fracture. Of potential relevance, zonisamide treatment was associated with reductions in serum phosphorus and increases in serum alkaline phosphatase, changes that may be related to metabolic acidosis and osteomalacia (see
Chronic, untreated metabolic acidosis in pediatric patients may reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of zonisamide on growth and bone-related sequelae has not been systematically investigated.
Measurement of baseline and periodic serum bicarbonate during treatment is recommended. If metabolic acidosis develops and persists, consideration should be given to reducing the dose or discontinuing zonisamide (using dose tapering). If the decision is made to continue patients on zonisamide in the face of persistent acidosis, alkali treatment should be considered.
Serum bicarbonate was not measured in the adjunctive controlled trials of adults with epilepsy. However, serum bicarbonate was studied in three clinical trials for indications which have not been approved: a placebo-controlled trial for migraine prophylaxis in adults, a controlled trial for monotherapy in epilepsy in adults, and an open label trial for adjunctive treatment of epilepsy in pediatric patients (3-16 years). In adults, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses of 100 mg to nearly 4 mEq/L at daily doses of 300 mg. In pediatric patients, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses from above 100 mg up to 300 mg, to nearly 4 mEq/L at daily doses from above 400 mg up to 600 mg.
In two controlled studies in adults, the incidence of a persistent treatment-emergent decrease in serum bicarbonate to less than 20 mEq/L (observed at 2 or more consecutive visits or the final visit) was dose-related at relatively low zonisamide doses. In the monotherapy trial of epilepsy, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 21% for daily zonisamide doses of 25 mg or 100 mg, and was 43% at a daily dose of 300 mg. In a placebo-controlled trial for prophylaxis of migraine, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 7% for placebo, 29% for 150 mg daily, and 34% for 300 mg daily. The incidence of persistent markedly abnormally low serum bicarbonate (decrease to less than 17 mEq/L and more than 5 mEq/L from a pretreatment value of at least 20 mEq/L) in these controlled trials was 2% or less.
In the pediatric study, the incidence of persistent, treatment-emergent decreases in serum bicarbonate to levels less than 20 mEq/L was 52% at doses up to 100 mg daily, was 90% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. The incidence of a persistent markedly abnormally low serum bicarbonate value was 4% at doses up to 100 mg daily, was 18% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. Some patients experienced moderately severe serum bicarbonate decrements down to a level as low as 10 mEq/L.
The relatively high frequencies of varying severities of metabolic acidosis observed in this study of pediatric patients (compared to the frequency and severity observed in various clinical trial development programs in adults) suggest that pediatric patients may be more likely to develop metabolic acidosis than adults.
4.5Seizures on Withdrawal:
As with other AEDs, abrupt withdrawal of ZONEGRAN in patients with epilepsy may precipitate increased seizure frequency or status epilepticus. Dose reduction or discontinuation of zonisamide should be done gradually.
4.6Teratogenicity:
Women of child bearing potential who are given zonisamide should be advised to use effective contraception. Zonisamide was teratogenic in mice, rats, and dogs and embryolethal in monkeys when administered during the period of organogenesis. A variety of fetal abnormalities, including cardiovascular defects, and embryo-fetal deaths occurred at maternal plasma levels similar to or lower than therapeutic levels in humans. These findings suggest that the use of ZONEGRAN during pregnancy in humans may present a significant risk to the fetus (see
4.7Cognitive/Neuropsychiatric Adverse Events:
Use of ZONEGRAN was frequently associated with central nervous system-related adverse events. The most significant of these can be classified into three general categories: 1) psychiatric symptoms, including depression and psychosis, 2) psychomotor slowing, difficulty with concentration, and speech or language problems, in particular, word-finding difficulties, and 3) somnolence or fatigue.
In placebo-controlled trials, 2.2% of patients discontinued ZONEGRAN or were hospitalized for depression compared to 0.4% of placebo patients. Among all epilepsy patients treated with ZONEGRAN, 1.4% were discontinued and 1.0% were hospitalized because of reported depression or suicide attempts. In placebo-controlled trials, 2.2% of patients discontinued ZONEGRAN or were hospitalized due to psychosis or psychosis-related symptoms compared to none of the placebo patients. Among all epilepsy patients treated with ZONEGRAN, 0.9% were discontinued and 1.4% were hospitalized because of reported psychosis or related symptoms.
Psychomotor slowing and difficulty with concentration occurred in the first month of treatment and were associated with doses above 300 mg/day. Speech and language problems tended to occur after 6–10 weeks of treatment and at doses above 300 mg/day. Although in most cases these events were of mild to moderate severity, they at times led to withdrawal from treatment.
Somnolence and fatigue were frequently reported CNS adverse events during clinical trials with ZONEGRAN. Although in most cases these events were of mild to moderate severity, they led to withdrawal from treatment in 0.2% of the patients enrolled in controlled trials. Somnolence and fatigue tended to occur within the first month of treatment. Somnolence and fatigue occurred most frequently at doses of 300–500 mg/day. Patients should be cautioned about this possibility and special care should be taken by patients if they drive, operate machinery, or perform any hazardous task.
4.8Hyperammonemia and Encephalopathy:
Hyperammonemia and encephalopathy have been reported with the postmarketing use of zonisamide. Zonisamide treatment inhibits carbonic anhydrase activity, which may cause metabolic acidosis that is associated with an increased risk for developing hyperammonemia. Hyperammonemia resulting from zonisamide can also be asymptomatic.
5ADVERSE REACTIONS
The most common adverse reactions with ZONEGRAN (an incidence at least 4% greater than placebo) in controlled clinical trials and shown in descending order of frequency were somnolence, anorexia, dizziness, ataxia, agitation/irritability, and difficulty with memory and/or concentration.
In controlled clinical trials, 12% of patients receiving ZONEGRAN as adjunctive therapy discontinued due to an adverse reaction compared to 6% receiving placebo. Approximately 21% of the 1,336 patients with epilepsy who received ZONEGRAN in clinical studies discontinued treatment because of an adverse reaction. The most common adverse reactions leading to discontinuation were somnolence, fatigue and/or ataxia (6%), anorexia (3%), difficulty concentrating (2%), difficulty with memory, mental slowing, nausea/vomiting (2%), and weight loss (1%). Many of these adverse reactions were dose-related (see
5.1Adverse Reaction Incidence in Controlled Clinical Trials:
Table 4 lists adverse reactions that occurred in at least 2% of patients treated with ZONEGRAN in controlled clinical trials that were numerically more common in the ZONEGRAN group. In these studies, either ZONEGRAN or placebo was added to the patient's current AED therapy.
5.2Other Adverse Reactions in Clinical Trials:
ZONEGRAN has been administered to 1,598 individuals during all clinical trials, only some of which were placebo-controlled. The frequencies represent the proportion of the 1,598 individuals exposed to ZONEGRAN who experienced an event on at least one occasion. All events are included except those already listed in the previous table or discussed in
Events are further classified within each category and listed in order of decreasing frequency as follows:
Body as a Whole: Frequent: Accidental injury, asthenia. Infrequent: Chest pain, flank pain, malaise, allergic reaction, face edema, neck rigidity. Rare: Lupus erythematosus.
Cardiovascular: Infrequent: Palpitation, tachycardia, vascular insufficiency, hypotension, hypertension, thrombophlebitis, syncope, bradycardia. Rare: Atrial fibrillation, heart failure, pulmonary embolus, ventricular extrasystoles.
Digestive: Frequent: Vomiting. Infrequent: Flatulence, gingivitis, gum hyperplasia, gastritis, gastroenteritis, stomatitis, cholelithiasis, glossitis, melena, rectal hemorrhage, ulcerative stomatitis, gastro-duodenal ulcer, dysphagia, gum hemorrhage. Rare: Cholangitis, hematemesis, cholecystitis, cholestatic jaundice, colitis, duodenitis, esophagitis, fecal incontinence, mouth ulceration.
Hematologic and Lymphatic: Infrequent: Leukopenia, anemia, immunodeficiency, lymphadenopathy. Rare: Thrombocytopenia, microcytic anemia, petechia.
Metabolic and Nutritional: Infrequent: Peripheral edema, weight gain, edema, thirst, dehydration. Rare: Hypoglycemia, hyponatremia, lactic dehydrogenase increased, SGOT increased, SGPT increased.
Musculoskeletal: Infrequent: Leg cramps, myalgia, myasthenia, arthralgia, arthritis.
Nervous System: Frequent: Tremor, convulsion, abnormal gait, hyperesthesia, incoordination. Infrequent: Hypertonia, twitching, abnormal dreams, vertigo, libido decreased, neuropathy, hyperkinesia, movement disorder, dysarthria, cerebrovascular accident, hypotonia, peripheral neuritis, reflexes increased. Rare: Dyskinesia, dystonia, encephalopathy, facial paralysis, hypokinesia, hyperesthesia, myoclonus, oculogyric crisis.
Behavioral Abnormalities –Non-Psychosis-Related: Infrequent: Euphoria.
Respiratory: Frequent: Pharyngitis, cough increased. Infrequent: Dyspnea. Rare: Apnea, hemoptysis.
Skin and Appendages: Frequent: Pruritus. Infrequent: Maculopapular rash, acne, alopecia, dry skin, sweating, eczema, urticaria, hirsutism, pustular rash, vesiculobullous rash.
Special Senses: Frequent: Amblyopia, tinnitus. Infrequent: Conjunctivitis, parosmia, deafness, visual field defect, glaucoma. Rare: Photophobia, iritis.
Urogenital: Infrequent: Urinary frequency, dysuria, urinary incontinence, hematuria, impotence, urinary retention, urinary urgency, amenorrhea, polyuria, nocturia. Rare: Albuminuria, enuresis, bladder pain, bladder calculus, gynecomastia, mastitis, menorrhagia.
6POST MARKETING EXPERIENCE
The following serious adverse reactions have been reported since approval and use of ZONEGRAN worldwide. These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure.
Acute pancreatitis, rhabdomyolysis, increased creatine phosphokinase, drug reaction with eosinophilia and systemic symptoms (DRESS), acute myopia and secondary angle closure glaucoma, and hyperammonemia and encephalopathy (see
To report SUSPECTED ADVERSE REACTIONS, contact Advanz Pharma (US) Corp. at 1-877-370-1142 or the FDA at 1-800-FDA-1088
7DRUG ABUSE AND DEPENDENCE
The abuse and dependence potential of ZONEGRAN has not been evaluated in human studies (see
8DOSAGE AND ADMINISTRATION
ZONEGRAN (zonisamide) is recommended as adjunctive therapy for the treatment of partial seizures in adults. Safety and efficacy in pediatric patients below the age of 16 have not been established. ZONEGRAN should be administered once or twice daily, using 25 mg or 100 mg capsules. ZONEGRAN is given orally and can be taken with or without food. Capsules should be swallowed whole.
8.1Adults over Age 16:
The prescriber should be aware that, because of the long half-life of zonisamide, up to two weeks may be required to achieve steady state levels upon reaching a stable dose or following dosage adjustment. Although the regimen described below is one that has been shown to be tolerated, the prescriber may wish to prolong the duration of treatment at the lower doses in order to fully assess the effects of zonisamide at steady state, noting that many of the side effects of zonisamide are more frequent at doses of 300 mg per day and above. Although there is some evidence of greater response at doses above 100–200 mg/day, the increase appears small and formal dose-response studies have not been conducted.
The initial dose of ZONEGRAN should be 100 mg daily. After two weeks, the dose may be increased to 200 mg/day for at least two weeks. It can be increased to 300 mg/day and 400 mg/day, with the dose stable for at least two weeks to achieve steady state at each level. Evidence from controlled trials suggests that ZONEGRAN doses of 100–600 mg/day are effective, but there is no suggestion of increasing response above 400 mg/day (see
8.2Patients with Renal or Hepatic Disease:
Because zonisamide is metabolized in the liver and excreted by the kidneys, patients with renal or hepatic disease should be treated with caution, and might require slower titration and more frequent monitoring (see
9HOW SUPPLIED
ZONEGRAN is available as 25 mg and 100 mg two-piece hard gelatin capsules. The capsules are printed in black with “ZONEGRAN 25” or “ZONEGRAN 100,” respectively. ZONEGRAN is available in bottles of 100 with strengths and colors as follows:
Store at 25°C (77°F), excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature], in a dry place and protected from light.
Manufactured for:
ZONEGRAN® is a registered trademark of Mercury Pharma Group Limited in the United States and Puerto Rico.
Revised: 11/2023
10Medication Guide
ZONEGRAN
(zonisamide) capsules
What is the most important information I should know about ZONEGRAN?
ZONEGRAN may cause serious side effects, including:
- Serious skin rash that can cause death.
- Serious allergic reactions that may affect different parts of the body.
- Less sweating and increase in your body temperature (fever).
- Serious eye problems
- Suicidal thoughts or actions in some people.
- Increased level of acid in your blood (metabolic acidosis).
- Problems with your concentration, attention, memory, thinking, speech, or language.
- Blood cell changes such as reduced red and white blood cell counts.
These serious side effects are described below.
- ZONEGRAN may cause a serious skin rash that can cause death. These serious skin reactions are more likely to happen when you begin taking ZONEGRAN within the first 4 months of treatment but may occur at later times.
- ZONEGRAN can cause other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your health care provider right away if you have:
- ZONEGRAN may cause you to sweat less and to increase your body temperature (fever). You may need to be hospitalized for this. You should watch for decreased sweating and fever, especially when it is hot and especially in children taking ZONEGRAN.
- ZONEGRAN may cause eye problems. Serious eye problems include:
- Like other antiepileptic drugs, ZONEGRAN may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
- ZONEGRAN can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones and can slow the rate of growth in children. Metabolic acidosis can happen with or without symptoms.
- ZONEGRAN may cause problems with your concentration, attention, memory, thinking, speech, or language.
- ZONEGRAN can cause blood cell changes such as reduced red and white blood cell counts. Call your healthcare provider if you develop fever, sore throat, sores in your mouth, or unusual bruising.
ZONEGRAN can have other serious side effects. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you. Be sure to read the section titled "What are the possible side effects of ZONEGRAN?"
What is ZONEGRAN?
ZONEGRAN is a prescription medicine that is used with other medicines to treat partial seizures in adults.
It is not known if ZONEGRAN is safe or effective in children under 16 years of age.
Do not take ZONEGRAN:
Do not take ZONEGRAN if you are allergic to medicines that contain sulfa.
Before taking ZONEGRAN, tell your healthcare provider about all your medical conditions, including if you:
ZONEGRAN is a prescription medicine that is used with other medicines to treat partial seizures in adults.
It is not known if ZONEGRAN is safe or effective in children under 16 years of age.
Do not take ZONEGRAN:
Do not take ZONEGRAN if you are allergic to medicines that contain sulfa.
Before taking ZONEGRAN, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have kidney problems
- have liver problems
- have a history of metabolic acidosis (too much acid in your blood)
- have weak, brittle bones or soft bones (osteomalacia, osteopenia or osteoporosis)
- have a growth problem
- are on a diet high in fat called a ketogenic diet
- have diarrhea
- have high blood levels of ammonia
Tell your healthcare provider if you:
- are pregnant or plan to become pregnant. ZONEGRAN may harm your unborn baby. Women who can become pregnant should use effective birth control. Tell your healthcare provider right away if you become pregnant while taking ZONEGRAN.
- are breastfeeding or plan to breastfeed. ZONEGRAN can pass into your breast milk. It is not known if ZONEGRAN in your breast milk can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take ZONEGRAN.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I take ZONEGRAN?
- Take ZONEGRAN exactly as prescribed. Your healthcare prescriber may change your dose. Your healthcare provider will tell you how much ZONEGRAN to take.
- Take ZONEGRAN with or without food.
- Swallow the capsules whole.
- If you take too much ZONEGRAN, call your local Poison Control Center or go to the nearest emergency room right away.
- Do not stop taking ZONEGRAN without talking to your healthcare provider. Stopping ZONEGRAN suddenly can cause serious problems, including seizures that will not stop (status epilepticus).
What should I avoid while taking ZONEGRAN?
- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking ZONEGRAN until you talk to your health care provider. ZONEGRAN taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how ZONEGRAN affects you. ZONEGRAN can slow your thinking and motor skills.
What are the possible side effects of ZONEGRAN?
ZONEGRAN can cause serious side effects. See
Other serious side effects include:
- kidney stones. Back pain, stomach pain, or blood in your urine may mean you have kidney stones. Drink plenty of fluids while you take ZONEGRAN to lower your chance of getting kidney stones.
- problems with mood or thinking (new or worse depression; sudden changes in mood, behavior, or loss of contact with reality, sometimes associated with hearing voices or seeing things that are not really there; feeling sleepy or tired; trouble concentrating; speech and language problems). Call your healthcare provider right away if you have any of the symptoms listed above.
- high blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting.
The most common side effects of ZONEGRAN include:
- drowsiness
- loss of appetite
- dizziness
- problems with concentration or memory
- trouble with walking and coordination
- agitation or irritability
Side effects can happen at any time, but are more likely to happen during the first several weeks after starting ZONEGRAN.
These are not all of the possible side effects of ZONEGRAN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZONEGRAN?
- Store ZONEGRAN between 59°F to 86°F (15°C to 30°C)
- Keep ZONEGRAN dry and away from light
Keep ZONEGRAN and all medicines out of the reach of children.
General Information about the safe and effective use of ZONEGRAN
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZONEGRAN for a condition for which it was not prescribed. Do not give ZONEGRAN to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZONEGRAN that is written for health professionals.
What are the ingredients in ZONEGRAN?
Active ingredient: zonisamide
Inactive ingredients in ZONEGRAN 25 mg capsules: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide
Inactive ingredients in ZONEGRAN 100 mg capsules: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide, FD&C Red No. 40 and FD&C Yellow No. 6
Manufactured for:
ZONEGRAN® is a registered trademark of Mercury Pharma Group Limited in the United States and Puerto Rico.
Distributed by Advanz Pharma (US) Corp. under license.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: November 2023