Brand Name
Lumisight
Generic Name
Pegulicianine
View Brand Information FDA approval date: April 17, 2024
Form: Injection
What is Lumisight (Pegulicianine)?
LUMISIGHT is indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery. LUMISIGHT is an optical imaging agent indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
WARNING: ANAPHYLAXIS AND OTHER SERIOUS HYPERSENSITIVITY REACTIONS
Serious hypersensitivity reactions, including anaphylaxis, can occur during or following administration of LUMISIGHT. Anaphylaxis occurred in 4/726 (0.6%) of patients in clinical studies. Signs and symptoms associated with other hypersensitivity reactions included pruritus, urticaria, hypotension, lip swelling, erythema, anxiety, chest pain, cyanosis, dizziness, dyspnea, headache, hypoesthesia, hyperventilation, maculopapular rash, nausea, paresthesia, visual changes, and vomiting.
- Before LUMISIGHT administration, assess all patients for any history of hypersensitivity reaction to contrast media or products containing polyethylene glycol (PEG).
- Always have emergency resuscitation drugs, equipment, and trained personnel promptly available.
- Monitor all patients for hypersensitivity reactions. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy.
- LUMISIGHT is contraindicated in patients with a history of hypersensitivity reactions to pegulicianine
1INDICATIONS AND USAGE
LUMISIGHT is indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery.
2DOSAGE FORMS AND STRENGTHS
For injection: dark blue lyophilized powder in a single-dose vial delivering 39 mg pegulicianine after reconstitution.
3CONTRAINDICATIONS
LUMISIGHT is contraindicated in patients with a history of hypersensitivity reaction to pegulicianine. Reactions have included anaphylaxis
4ADVERSE REACTIONS
The following clinically important adverse reactions are described elsewhere in the labeling:
- Anaphylaxis and Other Serious Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LUMISIGHT was evaluated in 726 patients who received a single dose of 1 mg/kg of LUMISIGHT. Among these 726 patients, 703 (97%) had breast cancer and 23 (3%) had other types of cancer. The mean age of the patients was 62 years (range: 36 years to 95 years), and 98% of them were female. Distribution by race was 82% White, 7% Black or African American, 6% Asian, and 5% other or unreported. Distribution by ethnicity was 3% Hispanic/Latino, 93% non-Hispanic/Latino, and 4% unknown or unreported.
Adverse reactions occurring in ≥ 1% of patients receiving LUMISIGHT were hypersensitivity (1.4%, including anaphylaxis [4 out of 726]) and chromaturia (85%). Chromaturia resolved within 48 hours after administration in 93% of patients, with the longest time to resolution of 15 days.
Adverse reactions occurring in use < 1% of patients were skin discoloration after extravasation, nausea, dyspnea, pyrexia, and vomiting.
5DRUG INTERACTIONS
Blue dyes used for SLN mapping procedures generate a fluorescent signal that interferes with the signal from LUMISIGHT when injected into the breast prior to imaging with LUMISIGHT. The potential of other dyes to interfere with LUMISIGHT imaging has not been evaluated. Avoid administration of dyes used for SLN mapping procedure before imaging the lumpectomy cavity in patients receiving LUMISIGHT.
6DESCRIPTION
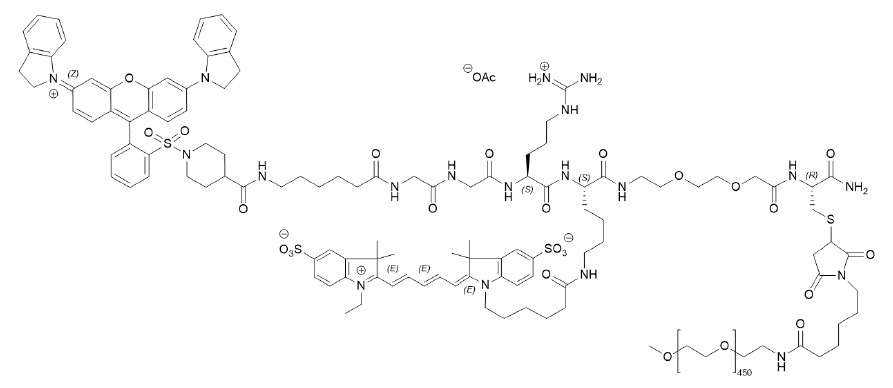
LUMISIGHT (pegulicianine for injection) is an optical imaging agent for intravenous use.
The chemical name of pegulicianine acetate is N-[6-(1-{2-[3,6-bis(2,3-dihydro-1H-indol-1-yl)xanthylium-9-yl]benzene-1-sulfonyl}piperidine-4-carboxamido)hexanoyl]glycylglycyl-L-arginyl-N6-(6-{2-[(1E,3E,5Z)-5-(1-ethyl-3,3-dimethyl-5-sulfonato-1,3-dihydro-2H-indol-2-ylidene)penta-1,3-dien-1-yl]-3,3-dimethyl-5-sulfonato-3H-indol-1-ium-1-yl}hexanoyl)-Llysyl-[2-(2-aminoethoxy)ethoxy]acetyl-S-[(3RS)-1-{6-[α-methylpoly(oxyethylene)-ω-amino]-6-oxohexyl}-2,5-dioxopyrrolidin-3-yl]-L-cysteinamide acetate with a molecular formula of C
Figure 1: Molecular structure of pegulicianine acetate
LUMISIGHT is supplied as a sterile, dark blue lyophilized powder. Each vial contains 40 mg of pegulicianine (equivalent to 40.1 mg of pegulicianine acetate) and the following inactive ingredients: 3.2 mg of dibasic sodium phosphate heptahydrate, 39.5 mg of mannitol, and 3.8 mg of monobasic sodium phosphate monohydrate to permit withdrawal of 3.9 mL of pegulicianine 10 mg/mL upon reconstitution with 4 mL of 0.45% sodium chloride injection, USP. The pH of the reconstituted solution is 6 to 7.
7CLINICAL STUDIES
The efficacy and safety of LUMISIGHT for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery in patients with breast cancer were evaluated in a randomized, multicenter, intra-patient controlled clinical trial NCT03686215. A total of 406 adult patients with confirmed invasive breast cancer, ductal carcinoma in situ (DCIS), or both received 1 mg/kg LUMISIGHT by intravenous injection 2 hours to 6 hours prior to imaging with the Lumicell DVS. Among them, 357 patients underwent LUMISIGHT-guided imaging after completion of the standard of care lumpectomy procedure. Patients who received neoadjuvant chemotherapy or radiotherapy prior to surgery were excluded from study. After the standard of care (SOC) lumpectomy was completed, the lumpectomy cavity was divided into six regions based on anatomic orientation and each region was imaged with the Lumicell DVS. When positive fluorescence signal was detected, the tissue was resected with a cavity shave procedure and the region was re-imaged. A maximum of two LUMISIGHT-guided shaves were obtained from a single region. Histopathology analysis of excised tissue served as the reference standard. Cavity regions that did not have a LUMISIGHT-guided cavity shave or tissue from a second surgery for a reference standard were assigned the margin status of the corresponding outermost surface of the lumpectomy specimen or SOC cavity shave, with a positive margin defined as tumor on ink for invasive cancers (with or without DCIS) or within 2 mm of the inked margin for DCIS alone.
The mean age of patients was 62 years (range: 36 years to 83 years). Distribution by race was 82% White, 7% Black or African American, 6% Asian, and 5% other. Distribution by ethnicity was 3% Hispanic/Latino, 94% non-Hispanic/Latino, and 3% unknown or unreported. In the study group, 70% of patients had invasive ductal carcinoma (with or without DCIS), 20% had DCIS only, 10% had invasive lobular carcinoma (with or without DCIS), and <1% had both invasive ductal and invasive lobular carcinoma.
The study assessed the proportion of patients receiving LUMISIGHT who had residual cancer detected and removed using the Lumicell DVS after completion of SOC lumpectomy. One hundred and sixty-six (166) of 357 (46%) patients had at least one LUMISIGHT-guided shave. A total of 27 of 357 patients had residual cancer confirmed by histopathology in at least one LUMISIGHT-guided shave (7.6%; 95% CI: 5.0%, 10.8%).
Table 1 shows sensitivity and specificity of LUMISIGHT for residual breast cancer after completion of SOC lumpectomy calculated from the 2,346 evaluable images in the study. Regions of the lumpectomy cavity from which LUMISIGHT-guided shaves were taken contributed more than one image to the analysis.
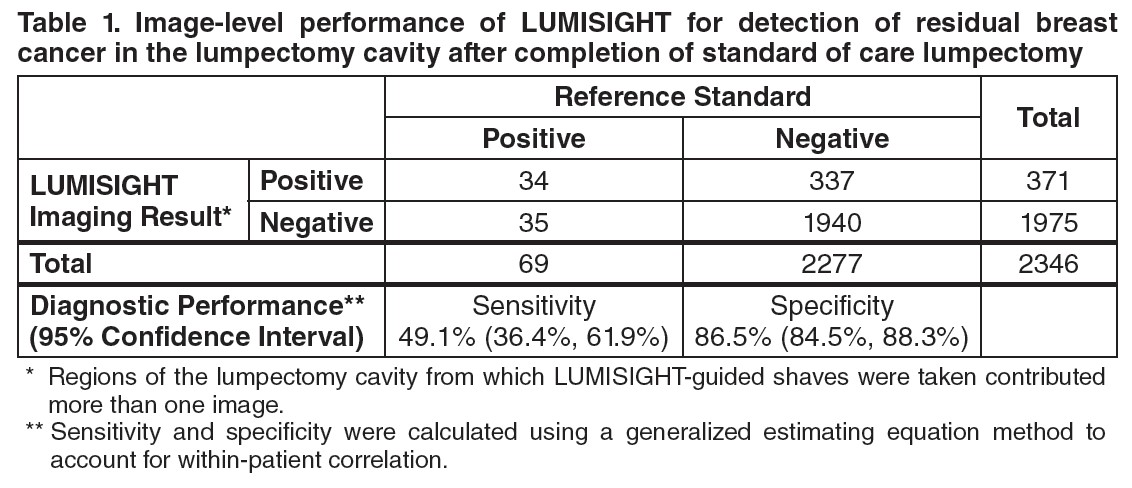
A total of 155 of 357 (43%) patients had at least one false positive image, and 28 of 357 (8%) patients had at least one false negative image. Among the 166 patients with at least one LUMISIGHT-guided shave, the mean total LUMISIGHT-guided shave volume was 22 cm
After completion of SOC lumpectomy, prior to LUMISIGHT-guided tissue removal, 62 of 357 patients (17%) had at least one cancer-positive margin. After LUMISIGHT-guided surgery, 9 of these 62 (15%) patients changed to having all cancer-negative margins, and 2 of the remaining 295 (1%) patients changed from having all cancer-negative margins to having at least one cancer-positive margin.
8HOW SUPPLIED/STORAGE AND HANDLING
How supplied
LUMISIGHT (pegulicianine) for injection is supplied as a dark blue lyophilized powder for reconstitution in a clear, glass single-dose vial in cartons of 10 vials (NDC 82292-040-10). After reconstitution, each vial delivers 39 mg pegulicianine.
Storage and Handling
Store vials of LUMISIGHT frozen at -25°C to -15°C (-13°F to 5°F) in the original carton to protect from light.
9PATIENT COUNSELING INFORMATION
Anaphylaxis and Other Serious Hypersensitivity Reactions
Inform patients of the risk of hypersensitivity reactions, including anaphylaxis, and instruct them to alert healthcare providers immediately if they experience signs and symptoms of a hypersensitivity reaction
Urine Discoloration
Inform patients that LUMISIGHT may cause blue discoloration of the urine that in most cases will resolve within 48 hours
Skin Discoloration After Extravasation
Inform patients that if extravasation occurs, it may result in blue discoloration of the skin at the injection site that will be evident for several weeks
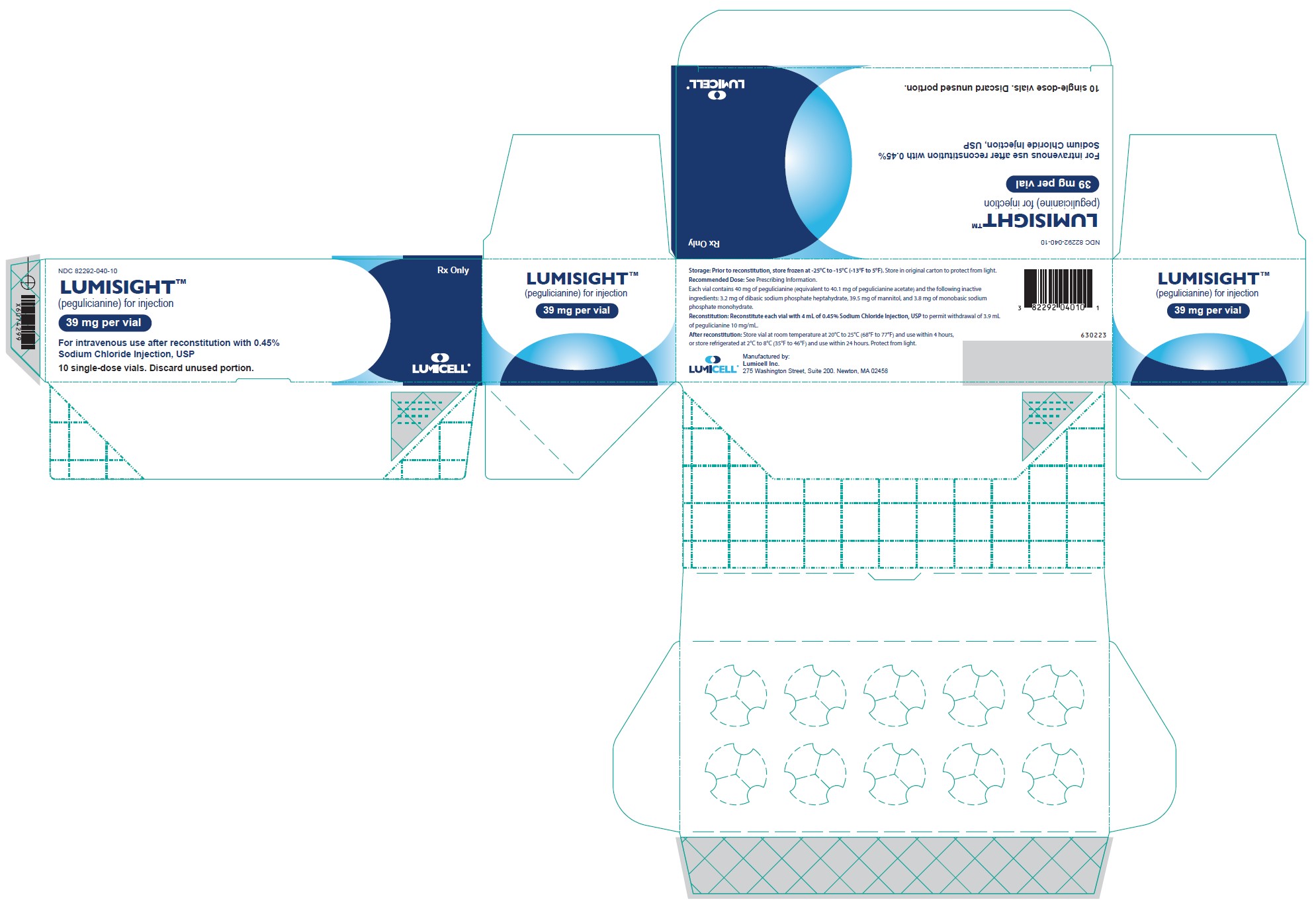
10PRINCIPAL DISPLAY PANEL - Vial Label
Rx Only
LUMISIGHT
Single-Dose vial.
For intravenous use after reconstitution with
After reconstitution, store at room temperature 20°C to 25°C
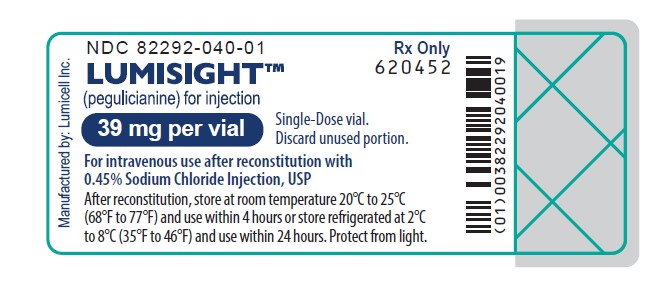
11PRINCIPAL DISPLAY PANEL - Carton Label
Rx Only
LUMISIGHT
Single-Dose vial.
For intravenous use after reconstitution with
10 single-dose vials. Discard unused portion.
Storage: Prior to reconstitution, store frozen at -25°C to -15°C (-13°F to 5°F). Store in original carton to protect from light.
Recommended Dose: See Prescribing Information.
Each vial contains 40 mg of pegulicianine (equivalent to 40.1 mg of pegulicianine acetate) and the following inactive
Reconstitution: Reconstitute each vial with 4 mL of 0.45% Sodium Chloride Injection, USP to permit withdrawal of 3.9 mL
of pegulicianine 10 mg/mL.
of pegulicianine 10 mg/mL.
After reconstitution: Store vial at room temperature at 20°C to 25°C (68°F to 77°F) and use within 4 hours,
or store refrigerated at 2°C to 8°C (35°F to 46°F) and use within 24 hours. Protect from light.
or store refrigerated at 2°C to 8°C (35°F to 46°F) and use within 24 hours. Protect from light.