Aczone
What is Aczone (Dapsone)?
For many people, acne is more than just a cosmetic issue, it can affect confidence, comfort, and quality of life. Persistent breakouts and skin inflammation can be frustrating, especially when over-the-counter products don’t seem to work. Aczone (dapsone) offers an effective, dermatologist-recommended solution for those struggling with acne that resists standard treatments.
Aczone is a topical prescription medication belonging to a class of drugs known as sulfonamides, which have antibacterial and anti-inflammatory properties. It helps treat acne by targeting both the bacteria and inflammation that contribute to breakouts. Approved by the U.S. Food and Drug Administration (FDA), Aczone is commonly used as a maintenance or adjunct therapy for moderate acne. Available in gel form, it is typically well-tolerated and can fit easily into daily skincare routines, helping patients achieve clearer, healthier-looking skin over time.
What does Aczone do?
Aczone is primarily used to treat acne vulgaris, the most common type of acne affecting teens and adults. Acne develops when pores become clogged with oil, dead skin cells, and bacteria, leading to pimples, blackheads, or inflamed lesions.
The active ingredient in Aczone, dapsone, helps reduce these symptoms by targeting both the bacterial and inflammatory aspects of acne. When applied regularly, Aczone can:
- Decrease the number and severity of acne lesions
- Reduce redness, swelling, and tenderness
- Help prevent new breakouts
Clinical studies have shown that Aczone can significantly improve acne symptoms after consistent use, with noticeable results often seen within 8 to 12 weeks (NIH, 2024). For many patients, it becomes an essential part of a long-term acne management plan that supports clearer skin and improved confidence.
While Aczone is mainly known for acne treatment, oral forms of dapsone are also used for other conditions such as dermatitis herpetiformis (a skin condition linked to celiac disease) and certain bacterial infections. However, when used topically as Aczone gel, the medication acts mainly on the skin and has fewer systemic effects.
How does Aczone work?
Aczone works through a dual mechanism, it reduces both inflammation and bacterial growth on the skin.
Dapsone targets Propionibacterium acnes (now called Cutibacterium acnes), the bacteria that contribute to acne formation. By inhibiting bacterial enzyme activity, it helps control infection and prevents the buildup of pus and inflammation within clogged pores.
At the same time, dapsone acts as an anti-inflammatory agent, calming the immune response that can cause redness, swelling, and irritation around pimples. This combination helps improve both inflammatory acne (such as red, swollen pimples) and non-inflammatory acne (such as whiteheads and blackheads).
This mechanism matters clinically because acne is not caused by bacteria alone, inflammation plays an equally significant role. By addressing both causes simultaneously, Aczone helps promote smoother skin texture and a healthier complexion without the harshness often associated with stronger acne medications.
Aczone side effects
Aczone is generally well-tolerated when used as directed, but some people may experience mild or temporary side effects, particularly in the first few weeks of use.
Common side effects may include:
- Dryness or peeling at the application site
- Mild redness or irritation
- Itching or burning sensation
- Oiliness in the skin after application
These symptoms typically fade as the skin adjusts to the medication. Applying a gentle, non-comedogenic moisturizer can help minimize dryness or irritation.
Less common but serious side effects may include:
- Yellowing of the skin or eyes (signs of liver issues or hemolysis)
- Dark brown or grayish skin discoloration (methemoglobinemia)
- Fatigue or shortness of breath (possible blood-related reaction)
Serious side effects are rare, especially with topical use, but they are more likely in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency, a genetic condition that affects red blood cell metabolism. For this reason, doctors may screen patients for G6PD deficiency before prescribing Aczone.
Patients should seek immediate medical attention if they experience unusual tiredness, bluish lips or fingertips, shortness of breath, or severe skin discoloration, as these could signal a rare but serious reaction.
Aczone dosage
Aczone topical gel (5% or 7.5%) is applied once or twice daily to affected areas. Patients should cleanse and dry their face, then apply a thin layer to the entire affected area to prevent breakouts.
Because dapsone is absorbed through the skin, healthcare providers may recommend periodic blood tests, especially for patients with G6PD deficiency or other blood disorders, to monitor for rare side effects.
For best results, Aczone should be used consistently. Stopping too soon can allow symptoms to return. Do not combine Aczone with topical benzoyl peroxide at the same time of day, as this may cause temporary skin discoloration.
Does Aczone have a generic version?
Yes. Aczone (dapsone) is available in FDA-approved generic forms in the United States. These generics contain the same active ingredient, strength, and safety profile as the brand-name product. The generic dapsone gel is available in both 5% and 7.5% formulations and provides the same therapeutic benefits at a typically lower cost.
Generic dapsone gel is as effective and safe as Aczone, available by prescription, and potentially covered by insurance or patient assistance.
Conclusion
Aczone (dapsone) offers a powerful yet gentle solution for managing acne, especially for patients who have not found success with other treatments. By addressing both bacteria and inflammation, it helps reduce breakouts, prevent new acne formation, and improve skin clarity over time.
Aczone effectively treats acne, but follow doctor’s orders and report any unusual symptoms, especially with blood disorders or G6PD deficiency. Consistent use and dermatologic follow-ups ensure safety and efficacy, leading to clearer skin and increased confidence.
References
- U.S. Food and Drug Administration (FDA). (2024). Aczone (dapsone) gel prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Dapsone (topical route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Dapsone topical: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Topical dapsone for acne management. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
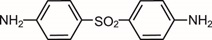
- Inform patients that methemoglobinemia can occur with topical dapsone treatment. Advise patients to seek immediate medical attention if they develop cyanosis
- Inform patients who have G6PD deficiency that hemolytic anemia may occur with topical dapsone treatment. Advise patients to seek medical attention if they develop signs and symptoms suggestive of hemolytic anemia
- Advise patients to apply ACZONE Gel, 7.5%, once daily to the entire face
- ACZONE Gel, 7.5% is for topical use only.
- Do not apply ACZONE Gel, 7.5% to eyes, mouth, or mucous membranes.