Kimmtrak
What is Kimmtrak (Tebentafusp)?
A diagnosis of a rare and aggressive cancer like uveal melanoma, especially once it has spread, can be a deeply frightening and uncertain time. For years, patients with this specific type of eye cancer had very few effective treatment options. However, a breakthrough in cancer research has brought a new wave of hope. This advancement comes in the form of Kimmtrak (tebentafusp-tebn).
Kimmtrak is a first-in-class prescription medication that represents a completely new way of fighting this disease. It is a type of cutting-edge immunotherapy known as a bispecific T-cell engager. It is not chemotherapy or traditional radiation; instead, it is a highly specialized, targeted therapy designed to harness the power of your own immune system to find and destroy cancer cells. For a specific group of patients with metastatic uveal melanoma, Kimmtrak is the first and only therapy that has been shown to help them live longer.
What does Kimmtrak do?
Kimmtrak is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with unresectable or metastatic uveal melanoma. This means it is used when the eye cancer cannot be removed with surgery or has spread to other parts of the body.
Importantly, Kimmtrak is not for every patient with this disease. It is a form of precision medicine for individuals who test positive for a specific genetic marker called HLA-A*02:01. This marker is a normal part of the immune system and is present in about half of the general population.
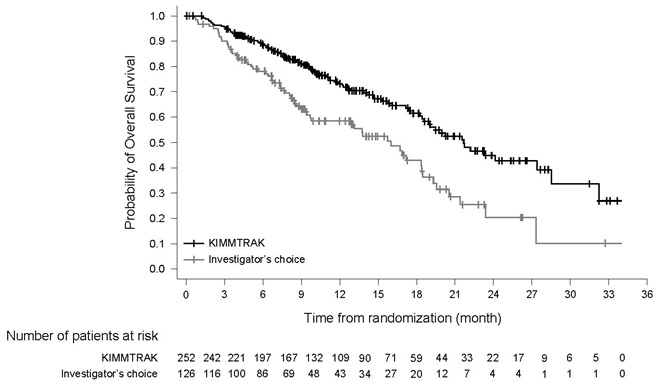
The primary goal of treatment with Kimmtrak is to improve overall survival. In the pivotal clinical trial that led to its approval, patients treated with Kimmtrak had a significantly better chance of living longer compared to patients who received other standard cancer therapies. It was the first therapy ever to show a survival benefit for patients with previously untreated metastatic uveal melanoma (Immunocore, 2022).
How does Kimmtrak work?
The way Kimmtrak works is a brilliant example of modern cancer immunotherapy. To understand it, think of your immune system’s T-cells as highly trained soldiers whose job is to find and eliminate threats like cancer cells. However, cancer cells are masters of disguise and can often hide from these soldiers.
Kimmtrak acts as a special “bridge” or a “matchmaker” that unmasks the cancer cells and forces the T-cells to attack. The Kimmtrak molecule is engineered to be bispecific, meaning it has two different “arms”:
- One arm is designed to grab onto a specific protein called gp100, which is found on the surface of the uveal melanoma cells.
- The other arm is designed to grab onto a protein called CD3, which is found on the surface of your T-cells.
By simultaneously binding to both the cancer cell and the T-cell, Kimmtrak physically brings the immune soldier directly to its target. This forced connection activates the T-cell, which then releases powerful toxins that destroy the melanoma cell. This unique mechanism redirects your body’s own immune system to specifically recognize and fight the cancer that it previously could not see.
Kimmtrak side effects
Because Kimmtrak powerfully activates the immune system, its side effects are primarily related to this widespread immune response.
Kimmtrak has a boxed warning, the FDA’s most serious type, for the risk of Cytokine Release Syndrome (CRS). CRS is a potentially serious and life-threatening condition that can occur when the immune system becomes over-activated. Symptoms can include fever, low blood pressure (hypotension), a low level of oxygen in the blood (hypoxia), chills, nausea, vomiting, fatigue, headache, and confusion.
The most common side effects of Kimmtrak include:
- Cytokine Release Syndrome
- Skin rash, itching, and redness
- Fever
- Nausea and vomiting
- Abdominal pain
- Fatigue
- Swelling (edema)
- Headache
- A drop in blood pressure
Initial treatment requires hospitalization for close monitoring due to CRS risk. Contact your doctor immediately for severe blistering or peeling skin rash. Your doctor will closely monitor all side effects, especially early in treatment.
Kimmtrak dosage
Kimmtrak is not a pill. It is administered as an intravenous (IV) infusion into a vein. The infusion itself usually takes about 15 to 20 minutes and is given once a week.
Due to high CRS risk, initial doses require strict safety monitoring. The first three infusions are hospital-administered, each followed by a mandatory 16-hour hospital stay for observation of vital signs, oxygen, and CRS. If severe side effects are absent after the third dose, weekly outpatient infusions may be possible. Regular blood tests, including liver function, will be ordered throughout treatment.
Does Kimmtrak have a generic version?
No, Kimmtrak (tebentafusp-tebn) is a highly complex, brand-name biologic medication. There is no generic or biosimilar version available. However, international versions may exist in other markets. As a first-in-class therapy, it is protected by patents that prevent other manufacturers from creating an equivalent for many years.
Conclusion
For patients with HLA-A*02:01-positive metastatic uveal melanoma, Kimmtrak represents a landmark achievement and a new standard of care. Its innovative approach of using the body’s own immune system to fight cancer has, for the first time, provided a proven survival benefit for this challenging disease.
While the treatment comes with the serious risk of Cytokine Release Syndrome and requires intensive monitoring in a hospital setting at the start, its potential benefits are profound. A thorough discussion with your oncology team will help you understand the risks and benefits. When administered under the care of an experienced medical team, Kimmtrak offers a powerful and hopeful path forward.
References
- Immunocore. (2022). KIMMTRAK® (tebentafusp-tebn) Prescribing Information. U.S. Food and Drug Administration. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761226s000lbl.pdf
- National Cancer Institute. (2022). FDA Approves Tebentafusp for Unresectable or Metastatic Uveal Melanoma. Retrieved from https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-tebentafusp-uveal-melanoma
- Mayo Clinic. (2024). Tebentafusp-tebn (Intravenous Route). Retrieved from https://www.mayoclinic.org/drugs-supplements/tebentafusp-tebn-intravenous-route/side-effects/drg-20524419
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a phase II open-label, single-arm, multi-center study of tebentafusp in HLA- A\*0201 positive previously untreated (1L) untreated metastatic uveal melanoma (mUM) with an integrated circulating tumor DNA (ctDNA) biomarker.
Summary: This Phase 2 study evaluates the efficacy and safety of sequential treatment with percutaneous hepatic perfusion (PHP) using melphalan/HDS followed by tebentafusp in patients with metastatic uveal melanoma (mUM) with isolated liver metastases. The rationale is that PHP enhances antigen release and immunomodulation, potentially sensitizing tumors to tebentafusp in HLA-A\*02:01-positive patients.
Summary: At least 50% of patients with high-risk primary uveal melanoma will develop a recurrence following treatment of the primary tumour. Observation is currently the standard of care in the non-metastatic setting. Tebentafusp is the first agent proven to improve overall survival in patients with metastatic uveal melanoma in a randomized trial. Based on the results in the advanced setting, it is hypothe...
Related Latest Advances
Brand Information
- Cytokine Release Syndrome [
- Skin Reactions [
- Elevated Liver Enzymes [
- One single-dose vial containing 100 mcg of tebentafusp-tebn in 0.5 mL of sterile, preservative-free, clear, colorless or slightly yellowish solution.
- Store KIMMTRAK vials in the original carton refrigerated at 2°C to 8°C (36°F to 46°F) and protect from light until time of use. Do not freeze. Do not shake.
- Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform females of the risk to a fetus
- Advise females of reproductive potential to use effective contraception while on KIMMTRAK and for 1 week after the last dose
- Advise patients not to breastfeed during treatment with KIMMTRAK and for 1 week after the last dose
Immunocore Limited
92 Park Drive, Milton Park
Abingdon, Oxfordshire
United Kingdom, OX144RY
License no: 2239
Baxter Oncology GmbH
Kantstraβe 2
33790 Halle/Westfalen
Germany
Immunocore Commercial LLC
181 Washington Street,
Conshohocken, PA, US





