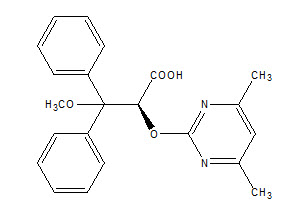
Letairis
What is Letairis (Ambrisentan)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Pulmonary arterial hypertension (PAH) is a rare, progressive, and potentially fatal disease characterized by increased pulmonary vascular resistance and right ventricular dysfunction. Among the four major molecular pathways involved in PAH pathophysiology-nitric oxide, prostacyclin, activin, and endothelin-1 (ET-1)-the endothelin pathway plays a central role. Endothelin-1 acts on ETA and ETB recep...
Summary: Application of Budesonide in Combination with Ambrisentan in the treatment of IgA nephropathy with progression ESKD risk (24-hour urinary protein ≥ 0.5g/24h), observing the degree and safety of reducing urinary protein and delaying eGFR progression, and observing changes in serum Gd-IgA1 levels
Summary: This is a multicenter, randomized, controlled, double-blind, and non-inferiority clinical trial to compare the efficacy of sequential to initial combination therapy in patients with pulmonary arterial hypertension (PAH). Ambrisentan and Tadalafil will be used in the study. Our research hypothesis is that the efficacy of sequential combination therapy in PAH patients is not inferior to the initial ...
Related Latest Advances
Brand Information
- To improve exercise ability and delay clinical worsening.
- In combination with tadalafil to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability
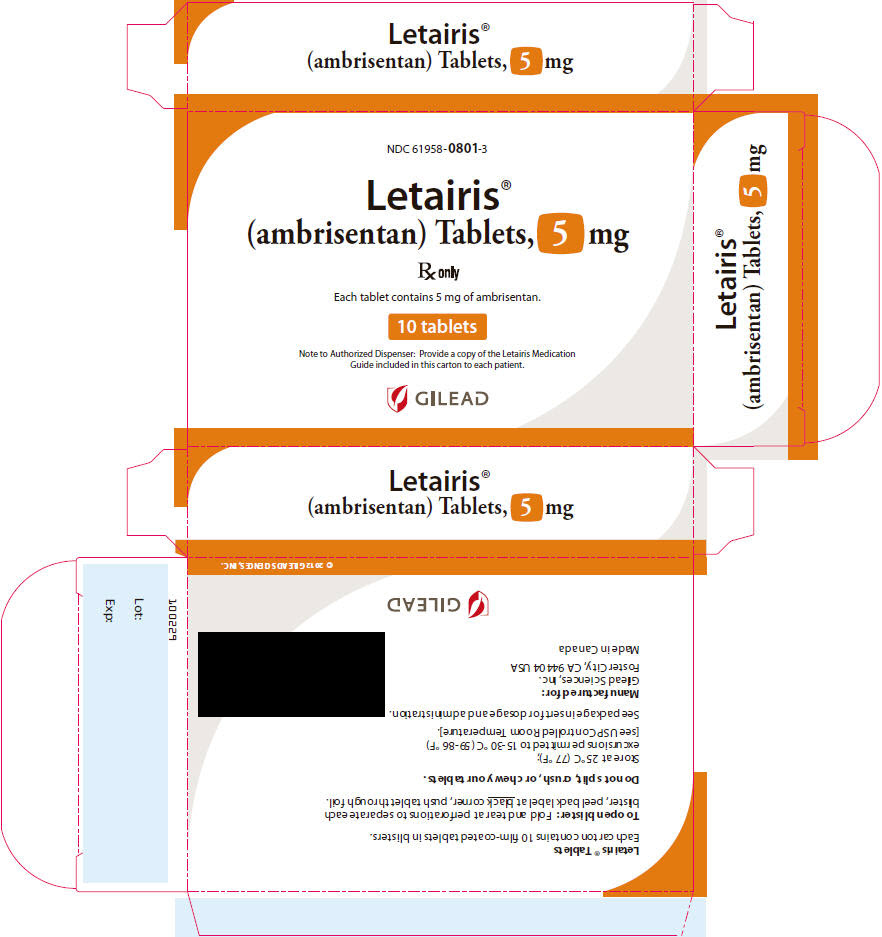
- Each 5 mg tablet is square convex, pale pink, with “5” on one side and “GSI” on the other side.
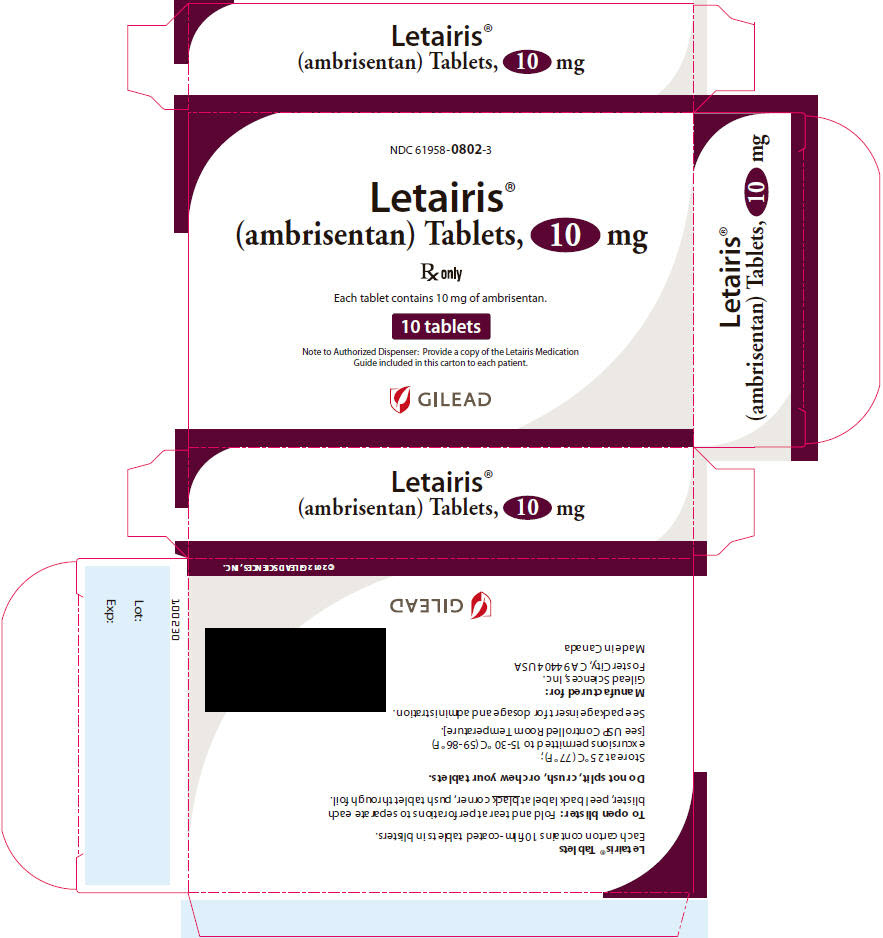
- Each 10 mg tablet is oval convex, deep pink, with “10” on one side and “GSI” on the other side.
- Embryo-fetal Toxicity
- Fluid Retention
- Pulmonary Edema with PVOD
- Decreased Sperm Count
- Hematologic Changes