Generic Name
Dichlorphenamide
Brand Names
Ormalvi, Keveyis
FDA approval date: December 13, 2021
Classification: Carbonic Anhydrase Inhibitor
Form: Tablet
What is Ormalvi (Dichlorphenamide)?
ORMALVI is indicated for the treatment of primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants. ORMALVI is an oral carbonic anhydrase inhibitor indicated for the treatment of primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ORMALVI (Dichlorphenamide)
1INDICATIONS AND USAGE
ORMALVI is indicated for the treatment of primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants.
2DOSAGE FORMS AND STRENGTHS
White to off-white, round shaped, flat faced beveled edged tablet, scored on one side, engraved with ‘ZD’ above the score and ‘50’ below the score, the other side is plain.
3CONTRAINDICATIONS
ORMALVI is contraindicated in the following circumstances:
- Hypersensitivity to dichlorphenamide or other sulfonamides
- Concomitant use of ORMALVI and high dose aspirin
- Severe pulmonary disease, limiting compensation to metabolic acidosis caused by ORMALVI
- Hepatic insufficiency: ORMALVI may aggravate hepatic encephalopathy.
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Hypersensitivity and Other Life-Threatening Reactions
- Hypokalemia
- Metabolic Acidosis
- Falls
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 9-week randomized controlled trial in adults with hyperkalemic or hypokalemic periodic paralysis (Study 1), the most common adverse reactions in patients treated with dichlorphenamide, with rates greater than placebo, were paresthesia, cognitive disorder, dysgeusia, and confusional state. The mean dose of dichlorphenamide was 94 mg/day in patients with hypokalemic periodic paralysis and 82 mg/day in patients with hyperkalemic periodic paralysis.
Table 1 lists the incidence of adverse reactions that occurred in 5% of patients treated with dichlorphenamide and more commonly than in patients treated with placebo in Study 1.
1Cognitive disorder combined cases with the preferred terms of cognitive disorder, disturbance in attention, and mental impairment.
4.2Postmarketing Experience
Adverse reactions have been identified during postapproval use of dichlorphenamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following are adverse reactions which have been reported during postapproval use of dichlorphenamide and were serious or are not reported in the previous section of labeling
5OVERDOSAGE
Symptoms of overdosage or toxicity may include drowsiness, anorexia, nausea, vomiting, dizziness, ataxia, tremor, and tinnitus.
In the event of overdosage, induce emesis or perform gastric lavage. The electrolyte disturbances most likely to be encountered from overdosage are hypokalemia and hyperchloremic metabolic acidosis.
6DESCRIPTION
ORMALVI tablets, USP contain dichlorphenamide, an oral carbonic anhydrase inhibitor.
Its empirical formula is C
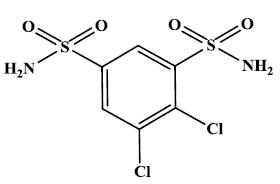
Dichlorphenamide USP is a white or practically white, crystalline powder with a molecular weight of 305.16. It is very slightly soluble in water but soluble in dilute solutions of alkali carbonate and hydroxide.
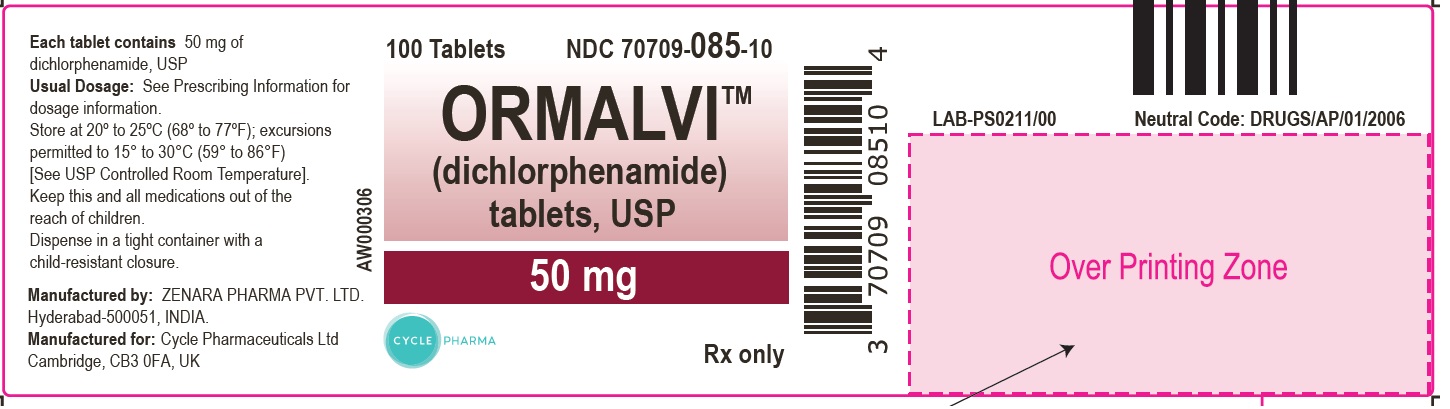
ORMALVI (dichlorphenamide) tablets, USP are supplied as tablets, for oral administration, each containing 50 mg dichlorphenamide. Inactive ingredients are lactose monohydrate, partially pregelatinized starch and magnesium stearate.
FDA approved dissolution test specifications differ from USP.
7CLINICAL STUDIES
The efficacy of dichlorphenamide was evaluated in two clinical studies, Study 1 and Study 2.
Study 1
Study 1 was a 9-week, double-blind, placebo-controlled multi-center study. Study 1 consisted of two substudies: a substudy in patients with hypokalemic periodic paralysis (n=44), and a substudy in patients with hyperkalemic periodic paralysis (n=21). The primary efficacy endpoint in both substudies was the average number of self-reported attacks of muscle weakness per week over the final 8 weeks of the trial. Withdrawal from the study for acute severe worsening (increase in attack frequency or severity) was also assessed as an endpoint.
In Study 1, the dose of dichlorphenamide was 50 mg b.i.d. for treatment-naïve patients. Patients already on dichlorphenamide prior to the study continued on the same dose while on dichlorphenamide during the study. In patients taking acetazolamide prior to the study, the dose of dichlorphenamide was set at 20% of the acetazolamide dose. Dose reduction for tolerability was permitted.
Hypokalemic Periodic Paralysis Substudy of Study 1
In the hypokalemic periodic paralysis substudy, median age of patients was 45 years and 73% of patients were male. Patients treated with dichlorphenamide (n=24) had 2.2 fewer attacks per week than patients (n=20) treated with placebo (p=0.02). None of the patients randomized to dichlorphenamide reached the endpoint of withdrawal from the study for acute worsening, vs. five patients randomized to placebo. The mean dose of dichlorphenamide at Week 9 was 94 mg/day.
Hyperkalemic Periodic Paralysis Substudy of Study 1
In the Hyperkalemic Periodic Paralysis substudy, median age of patients was 43 years and 43% of patients were male. During the double-blind treatment period, patients treated with dichlorphenamide (n=12) had 3.9 fewer attacks per week than patients (n=9) treated with placebo (p=0.08). None of the patients randomized to dichlorphenamide reached the endpoint of withdrawal from the study for acute worsening, vs. two patients randomized to placebo. The mean dose of dichlorphenamide at Week 9 was 82 mg/day.
Study 2
Study 2 was a 35-week, double-blind, placebo-controlled, multi-center, two-period crossover study. Study 2 also consisted of two substudies: a substudy in patients with hypokalemic periodic paralysis (n=42), and a substudy in patients with hyperkalemic periodic paralysis (n=31), including patients with Paramyotonia Congenita. The primary endpoint in the hypokalemic periodic paralysis substudy was the incidence of acute intolerable worsening (based on attack frequency or severity) necessitating withdrawal. The primary endpoint in the hyperkalemic periodic paralysis substudy was the average number of self-reported attacks of muscle weakness per week. Dosing was determined similarly to Study 1.
Hypokalemic Periodic Paralysis Substudy of Study 2
The hypokalemic periodic paralysis substudy included patients with a mean age of 38 years; 79% of patients were male. Acute intolerable worsening was observed in 2 patients on dichlorphenamide vs. 11 patients on placebo (p=0.02). The mean dose of dichlorphenamide at the end of the study was 96 mg/day.
Hyperkalemic Periodic Paralysis Substudy of Study 2
The hyperkalemic periodic paralysis substudy included patients with a mean age of 37 years; and 79% of patients were male. Patients treated had 2.3 fewer attacks per week on dichlorphenamide than on placebo (p=0.006). The mean dose of dichlorphenamide at the end of the study was 73 mg/day.
8HOW SUPPLIED/STORAGE AND HANDLING
Each ORMALVI (dichlorphenamide) tablet USP, 50 mg is white to off-white, round shaped, flat faced beveled edged tablet, scored on one side, engraved with ‘ZD’ above the score and ‘50’ below the score, the other side is plain.
ORMALVI (dichlorphenamide) tablets are supplied as follows:
Bottles of 100 NDC 70709-085-10
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
9PATIENT COUNSELING INFORMATION
Worsening of Symptoms
Advise patients to notify their physician if they experience acute worsening of symptoms of periodic paralysis.
Advise patients to notify their physician if they experience acute worsening of symptoms of periodic paralysis.
Hypersensitivity and Other Life-threatening Reactions
Inform patients that hypersensitivity and immune mediated reactions can occur with ORMALVI and could be fatal. Advise patients to discontinue ORMALVI and notify their healthcare provider immediately if they develop a rash or signs and symptoms of anaphylaxis or other life-threatening reactions [see Warnings and Precautions (
Inform patients that hypersensitivity and immune mediated reactions can occur with ORMALVI and could be fatal. Advise patients to discontinue ORMALVI and notify their healthcare provider immediately if they develop a rash or signs and symptoms of anaphylaxis or other life-threatening reactions [see Warnings and Precautions (
Drug Interactions
Instruct patients to notify their healthcare provider of all of the drugs and over-the-counter medications that they take and to not take aspirin or other salicylates without first discussing with their healthcare provider [see Drug Interactions (
Instruct patients to notify their healthcare provider of all of the drugs and over-the-counter medications that they take and to not take aspirin or other salicylates without first discussing with their healthcare provider [see Drug Interactions (
Metabolic Acidosis
Instruct patients to contact their healthcare provider immediately if they develop possible manifestations of metabolic acidosis (e.g., fast breathing, fatigue/tiredness, loss of appetite, or irregular heartbeat or palpitations) [see Warnings and Precautions (
Instruct patients to contact their healthcare provider immediately if they develop possible manifestations of metabolic acidosis (e.g., fast breathing, fatigue/tiredness, loss of appetite, or irregular heartbeat or palpitations) [see Warnings and Precautions (
Falls
Inform patients that ORMALVI can increase their risk of falls [see Warnings and Precautions (
Inform patients that ORMALVI can increase their risk of falls [see Warnings and Precautions (
Cognitive Impairment
Advise patients to notify their healthcare provider if they experience symptoms of cognitive impairment including confusion and memory lapse [see Adverse Reactions (
Advise patients to notify their healthcare provider if they experience symptoms of cognitive impairment including confusion and memory lapse [see Adverse Reactions (
Driving and Operating Machinery
ORMALVI may cause drowsiness/fatigue in some patients [see Adverse Reactions ( Caution patients on the potential for impaired ability to drive and operate machinery.
ORMALVI may cause drowsiness/fatigue in some patients [see Adverse Reactions ( Caution patients on the potential for impaired ability to drive and operate machinery.
10ORMALVITM(dichlorphenamide) 50 mg tablets, 100s Count