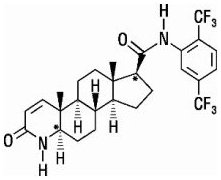
Avodart
What is Avodart (Dutasteride)?
Approved To Treat
Related Clinical Trials
Summary: This study is a double blinded RCT with pre-post test design on the effect of combination of dutasteride and aloe vera, compared to dutasteride only, on uroflowmetry results, prostate volume, and VEGF serum level among patients with benign prostate hyperplasia. The treatment was given for 12 weeks
Summary: Phase 2 clinical trial on the addition of dutasteride to combined androgen blockade (CAB) therapy in recurrent and/or metastatic (R/M) salivary duct carcinoma (SDC) patients. The study included two cohorts of patients: Cohort A, which comprises ADT-naïve patients, and Cohort B, which comprises ADT-resistant patients. Cohort A is closed for inclusion as of April 18, 2024.
Summary: This randomized clinical trial carried out on 60 Brazilian men, aged 18 to 65, with mild or moderate androgenetic alopecia. Two techniques for administering dutasteride will be compared, injected by syringes or needles or by needling through tattoo machines.
Related Latest Advances
Brand Information
- Pregnancy. Dutasteride use is contraindicated in women who are pregnant. In animal reproduction and developmental toxicity studies, dutasteride inhibited development of male fetus external genitalia. Therefore, AVODART may cause fetal harm when administered to a pregnant woman
- Patients with previously demonstrated clinically significant hypersensitivity (e.g., serious skin reactions, angioedema) to AVODART or other 5 alpha-reductase inhibitors