Generic Name
Miglustat
Brand Names
Yargesa, Opfolda, Zavesca
FDA approval date: July 31, 2003
Classification: Glucosylceramide Synthase Inhibitor
Form: Capsule
What is Yargesa (Miglustat)?
OPFOLDA is indicated, in combination with Pombiliti, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy . OPFOLDA is an enzyme stabilizer indicated, in combination with Pombiliti, a hydrolytic lysosomal glycogen-specific enzyme, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Yargesa (Miglustat)
1DOSAGE FORMS AND STRENGTHS
Capsules: 100 mg of miglustat, white opaque hard gelatin capsules with “709” printed in black on the body.
2CONTRAINDICATIONS
None
3ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In an open-label, active-controlled study, 36 adult type 1 Gaucher disease patients were treated with miglustat capsules, imiglucerase, or miglustat capsules plus imiglucerase [Study 3] for up to 12 months. Table 2 lists adverse reactions that occurred during the trial in greater than or equal to 5% of patients.
4DRUG INTERACTIONS
While co-administration of miglustat capsules appeared to increase the clearance of imiglucerase by 70%, these results are not conclusive because of the small number of patients studied and because patients took variable doses of imiglucerase [
5DESCRIPTION
YARGESA (miglustat capsules) is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most glycosphingolipids. YARGESA is an N-alkylated imino sugar, a synthetic analog of D-glucose. The chemical name for miglustat is 1,5-(butylimino)-1,5-dideoxy-D-glucitol with the chemical formula C
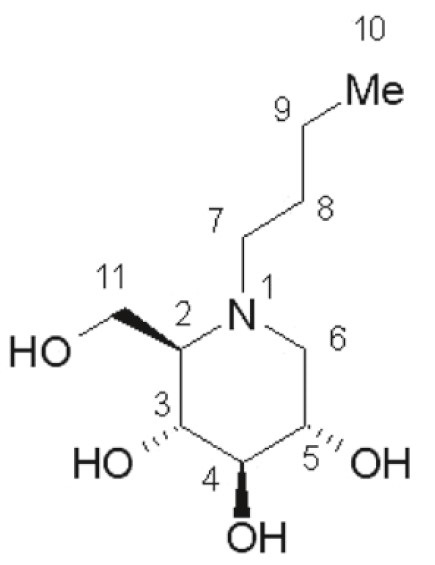
Miglustat is a white to off-white crystalline solid and has a bitter taste. It is highly soluble in water (greater than 1000 mg/mL as a free base).
6CLINICAL STUDIES
The efficacy of miglustat capsules in type 1 Gaucher disease has been investigated in two open-label, uncontrolled trials and one randomized, open-label, active-controlled trial with enzyme replacement given as imiglucerase. Patients who received miglustat capsules were treated with doses ranging from 100 to 600 mg a day, although the majority of patients were maintained on doses between 200 to 300 mg a day. Efficacy parameters included the evaluation of liver and spleen organ volume, hemoglobin concentration, and platelet count. A total of 80 patients were exposed to miglustat capsules during the three trials and their extension period.
Open-Label Uncontrolled Monotherapy Trials In Study 1, miglustat capsules was administered at a starting dose of 100 mg three times daily for 12 months (dose range of 100 once-daily to 200 mg three times daily) to 28 adult patients with type 1 Gaucher disease, who were unable to receive enzyme replacement therapy and who had not taken enzyme replacement therapy in the preceding 6 months. Twenty-two patients completed the trial. After 12 months of treatment, the results showed significant mean percent reductions from baseline in liver volume of 12% and spleen volume of 19%, a non-significant increase from baseline in mean absolute hemoglobin concentration of 0.26 g/dL and a mean absolute increase from baseline in platelet counts of 8 × 109/L (See Tables 3-6).
In Study 2, miglustat capsules was administered at a dose of 50 mg three times daily for 6 months to 18 adult patients with type 1 Gaucher disease who were unable to receive enzyme replacement therapy and who had not taken enzyme replacement therapy in the preceding 6 months. Seventeen patients completed the trial. After 6 months of treatment, the results showed significant mean percent reductions from baseline in liver volume of 6% and spleen volume of 5%. There was a non-significant mean absolute decrease from baseline in hemoglobin concentration of 0.13 g/dL and a non-significant mean absolute increase from baseline in platelet counts of 5 × 10 9/L (See Tables 3-6).
In Study 2, miglustat capsules was administered at a dose of 50 mg three times daily for 6 months to 18 adult patients with type 1 Gaucher disease who were unable to receive enzyme replacement therapy and who had not taken enzyme replacement therapy in the preceding 6 months. Seventeen patients completed the trial. After 6 months of treatment, the results showed significant mean percent reductions from baseline in liver volume of 6% and spleen volume of 5%. There was a non-significant mean absolute decrease from baseline in hemoglobin concentration of 0.13 g/dL and a non-significant mean absolute increase from baseline in platelet counts of 5 × 10 9/L (See Tables 3-6).
Extension Period Eighteen patients were enrolled in a 12-month extension to Study 1. A subset of patients continuing in the extension had larger mean baseline liver volumes, and lower mean baseline platelet counts and hemoglobin concentrations than the original study population (See Tables 3-6). After a total of 24 months of treatment, there were significant mean decreases from baseline in liver and spleen organ volumes of 15% and 27%, respectively, and significant mean absolute increases from baseline in hemoglobin concentration and platelet count of 0.9 g/dL and 14 × 10 9/L, respectively (See Tables 3-6).
Sixteen patients were enrolled in a 6-month extension to Study 2. After a total of 12 months of treatment, there was a mean decrease from baseline in spleen organ volume of 10%, whereas the mean percent decrease in liver organ volume remained at 6%. There were no significant changes in hemoglobin concentrations or platelet counts (See Tables 3-6).
Liver volume results from Studies 1 and 2 and their extensions are summarized in Table 3:
Sixteen patients were enrolled in a 6-month extension to Study 2. After a total of 12 months of treatment, there was a mean decrease from baseline in spleen organ volume of 10%, whereas the mean percent decrease in liver organ volume remained at 6%. There were no significant changes in hemoglobin concentrations or platelet counts (See Tables 3-6).
Liver volume results from Studies 1 and 2 and their extensions are summarized in Table 3:
Spleen volume results from Studies 1 and 2 and their extensions are summarized in Table 4:
Hemoglobin concentration results from Studies 1 and 2 and their extensions are summarized in Table 5:
Platelet count results from Studies 1 and 2 and their extensions are summarized in Table 6:
Open-Label Active-Controlled Trial Study 3 was an open-label, randomized, active-controlled study of 36 adult patients with type 1 Gaucher disease, who had been receiving enzyme replacement therapy with imiglucerase for a minimum of 2 years prior to study entry. Patients were randomized 1:1:1 to one of three treatment groups, as follows:
• miglustat capsules 100 mg three times daily alone
• imiglucerase (patient‘s usual dose) alone
• miglustat capsules 100 mg three times daily and imiglucerase (usual dose)
Patients were treated for 6 months, and 33 patients completed the study. Because miglustat capsules is only indicated as monotherapy, the results for the monotherapy arms are described below. At Month 6, the results showed a decrease in mean percent change in liver volume in the miglustat capsules treatment group compared to the imiglucerase alone group. There were no significant differences between the groups for mean absolute changes in liver and spleen volume and hemoglobin concentration. However, there was a significant difference between the miglustat capsules alone and imiglucerase alone groups in platelet counts at Month 6, with the miglustat capsules alone group having a mean absolute decrease in platelet count of 21.6 × 10 9/L and the imiglucerase alone group having a mean absolute increase in platelet count of 10.1 × 10 9/L (See Tables 7-10).
• miglustat capsules 100 mg three times daily alone
• imiglucerase (patient‘s usual dose) alone
• miglustat capsules 100 mg three times daily and imiglucerase (usual dose)
Patients were treated for 6 months, and 33 patients completed the study. Because miglustat capsules is only indicated as monotherapy, the results for the monotherapy arms are described below. At Month 6, the results showed a decrease in mean percent change in liver volume in the miglustat capsules treatment group compared to the imiglucerase alone group. There were no significant differences between the groups for mean absolute changes in liver and spleen volume and hemoglobin concentration. However, there was a significant difference between the miglustat capsules alone and imiglucerase alone groups in platelet counts at Month 6, with the miglustat capsules alone group having a mean absolute decrease in platelet count of 21.6 × 10 9/L and the imiglucerase alone group having a mean absolute increase in platelet count of 10.1 × 10 9/L (See Tables 7-10).
Extension period Twenty-nine patients were enrolled in a 6-month extension to Study 3. In the extension phase, all 29 patients had withdrawn from imiglucerase and received open-label miglustat capsules 100 mg three times daily monotherapy. At Month 12, the results showed non-significant decreases in platelet counts from baseline in all the treatment groups (by original randomization). There was a significant decrease in platelet counts from Month 6 to Month 12 in the group originally randomized to treatment with imiglucerase, and a continued decrease in platelet counts in the group originally randomized to miglustat capsules alone. There were no significant changes in any treatment group for liver volume, spleen volume, or hemoglobin concentration (See Tables 7-10).
Liver volume results from Study 3 and extension are summarized in Table 7:
Spleen volume results from Study 3 and extension are summarized in Table 8:
Hemoglobin concentration results from Study 3 and extension are summarized in Table 9:
Platelet count results from Study 3 and extension are summarized in Table 10:
Patients with platelet counts above 150 × 10
7HOW SUPPLIED/STORAGE AND HANDLING
YARGESA is supplied in hard gelatin capsules containing 100 mg miglustat. YARGESA 100 mg capsules are white opaque with “709” printed in black on the body.
Storage: Store at 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C to 30°C (59°F to 86°F) (see USP Controlled Room Temperature).
Keep out of reach of children
Keep out of reach of children
8PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Pregnancy Advise pregnant women and females of reproductive potential of the potential risk to a fetus, based on animal data. Advise patients who may become pregnant to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation Advise women not to breastfeed if they are taking YARGESA [see Use in Specific Populations (8.2)].
Manufactured for:
Edenbridge Pharmaceuticals, LLC
Edenbridge Pharmaceuticals, LLC
DBA Dexcel Pharma USA
Issued: July 2024
9Patient Information
YARGESA
What is YARGESA ? YARGESA is a prescription medicine used alone to treat adults with mild to moderate type 1 Gaucher disease.YARGESA is used only in people who cannot be treated with enzyme replacement therapy.
It is not known if YARGESA is safe and effective in children under 18 years of age.
It is not known if YARGESA is safe and effective in children under 18 years of age.
Before taking YARGESA , tell your healthcare provider about all of your medical conditions, including if you: • have kidney problems
• are pregnant or plan to become pregnant. Yargesa may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with YARGESA
• are breastfeeding or plan to breastfeed. It is not known if YARGESA passes into your breast milk and may harm your baby. Do not breastfeed during treatment with YARGESA. Talk to your healthcare provider about the best way to feed your baby during treatment with YARGESA.
• are pregnant or plan to become pregnant. Yargesa may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with YARGESA
• are breastfeeding or plan to breastfeed. It is not known if YARGESA passes into your breast milk and may harm your baby. Do not breastfeed during treatment with YARGESA. Talk to your healthcare provider about the best way to feed your baby during treatment with YARGESA.
Tell your Healthcare Provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. YARGESA may affect how other medicines work.
How should I take YARGESA ? • Take YARGESA exactly as your healthcare provider tells you to.
• Take YARGESA at the same time each day.
• If you miss a dose of YARGESA, skip that dose. Take the next miglustat capsule at the usual time.
• Take YARGESA at the same time each day.
• If you miss a dose of YARGESA, skip that dose. Take the next miglustat capsule at the usual time.
What are the possible side effects of YARGESA ? • Numbness, tingling, pain, or burning in your hands or feet (peripheral neuropathy). Call your healthcare provider right away if you get numbness, tingling, pain, or burning in your hands or feet.
• Your healthcare provider may test your nerves (neurological exam) before you start YARGESA and during treatment with YARGESA.
• New or worsening hand tremors (shaky movements).Tremors are common with YARGESA and may begin within the first month of starting treatment. Sometimes the tremors may go away between 1 to 3 months with continued treatment. Your healthcare provider may lower your dose or stop YARGESA if you develop new or worsening hand tremors. Call your healthcare provider right away if you get new hand tremors during treatment with YARGESA or if the hand tremors you already have get worse.
• Diarrheais common with YARGESA and sometimes can be serious. Your healthcare provider may prescribe another medicine (anti-diarrheal) to treat diarrhea if it is a problem for you and may recommend changes to your diet, such as avoiding foods high in carbohydrates. Talk with your healthcare provider about your diet if you have diarrhea.
• Weight lossis common with YARGESA and sometimes can be serious. You may lose weight when you start treatment with YARGESA.
• Low platelet countis common with YARGESA and can be serious. Your healthcare provider may do blood tests to monitor your blood platelet count.
The most common side effects of YARGESA include: • weight loss
• stomach pain
• gas
• nausea and vomiting
• headache, including migraine
• back pain
• constipation
• dry mouth
• heaviness in arms and legs
• memory loss
• unsteady walking
• leg cramps
• dizziness
• weakness
• vision problems
• muscle cramps
• loss of appetite
• indigestion
• numbness, tingling, pain, or burning of your skin
• stomach bloating
• stomach pain not related to food
• menstrual changes
• Your healthcare provider may test your nerves (neurological exam) before you start YARGESA and during treatment with YARGESA.
• New or worsening hand tremors (shaky movements).Tremors are common with YARGESA and may begin within the first month of starting treatment. Sometimes the tremors may go away between 1 to 3 months with continued treatment. Your healthcare provider may lower your dose or stop YARGESA if you develop new or worsening hand tremors. Call your healthcare provider right away if you get new hand tremors during treatment with YARGESA or if the hand tremors you already have get worse.
• Diarrheais common with YARGESA and sometimes can be serious. Your healthcare provider may prescribe another medicine (anti-diarrheal) to treat diarrhea if it is a problem for you and may recommend changes to your diet, such as avoiding foods high in carbohydrates. Talk with your healthcare provider about your diet if you have diarrhea.
• Weight lossis common with YARGESA and sometimes can be serious. You may lose weight when you start treatment with YARGESA.
• Low platelet countis common with YARGESA and can be serious. Your healthcare provider may do blood tests to monitor your blood platelet count.
The most common side effects of YARGESA include: • weight loss
• stomach pain
• gas
• nausea and vomiting
• headache, including migraine
• back pain
• constipation
• dry mouth
• heaviness in arms and legs
• memory loss
• unsteady walking
• leg cramps
• dizziness
• weakness
• vision problems
• muscle cramps
• loss of appetite
• indigestion
• numbness, tingling, pain, or burning of your skin
• stomach bloating
• stomach pain not related to food
• menstrual changes
These are not all the possible side effects of YARGESA. For more information, ask your healthcare provider or pharmacist.Call your doctor for medical advice about side effects.
How should I store YARGESA ? • Store YARGESA at room temperature between 68ºF to 77ºF (20ºC to 25ºC)
Keep YARGESA and all medicines out of the reach of children
Keep YARGESA and all medicines out of the reach of children
General information about the safe and effective use of YARGESA . Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
Do not use YARGESA for a condition for which it was not prescribed. Do not give YARGESA to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about YARGESA that is written for health professionals.
Do not use YARGESA for a condition for which it was not prescribed. Do not give YARGESA to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about YARGESA that is written for health professionals.
What are the ingredients in YARGESA ? : miglustat
Inactive ingredients:sodium starch glycolate (type A, potato), povidone (K-29/32), and magnesium stearate.
The capsule shell contains: gelatin and titanium dioxide; the edible printing ink contains black iron oxide, shellac,
and propylene glycol.
Inactive ingredients:sodium starch glycolate (type A, potato), povidone (K-29/32), and magnesium stearate.
The capsule shell contains: gelatin and titanium dioxide; the edible printing ink contains black iron oxide, shellac,
and propylene glycol.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Edenbridge Pharmaceuticals, LLC
Edenbridge Pharmaceuticals, LLC
DBA Dexcel Pharma USA
Revised: 07/2024
10PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
