Brand Name
Rivfloza
Generic Name
Nedosiran
View Brand Information FDA approval date: February 19, 2024
Form: Injection
What is Rivfloza (Nedosiran)?
RIVFLOZA is indicated to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 and relatively preserved kidney function, e.g., eGFR ≥30 mL/min.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
RIVFLOZA (nedosiran)
1INDICATIONS AND USAGE
RIVFLOZA is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, e.g., eGFR ≥30 mL/min/1.73 m
2DOSAGE FORMS AND STRENGTHS
RIVFLOZA Injection 160 mg/mL (present as 170 mg nedosiran sodium) is a clear, colorless-to-yellow solution available as follows:
- 80 mg/0.5 mL single-dose vial
- 128 mg/0.8 mL single-dose Pre-filled Syringe
- 160 mg/ mL single-dose Pre-filled Syringe
3CONTRAINDICATIONS
None.
4DESCRIPTION
RIVFLOZA injection contains nedosiran, a double-stranded small interfering RNA (siRNA) with four covalently attached
The structural formula of the nedosiran sodium drug substance is presented below:
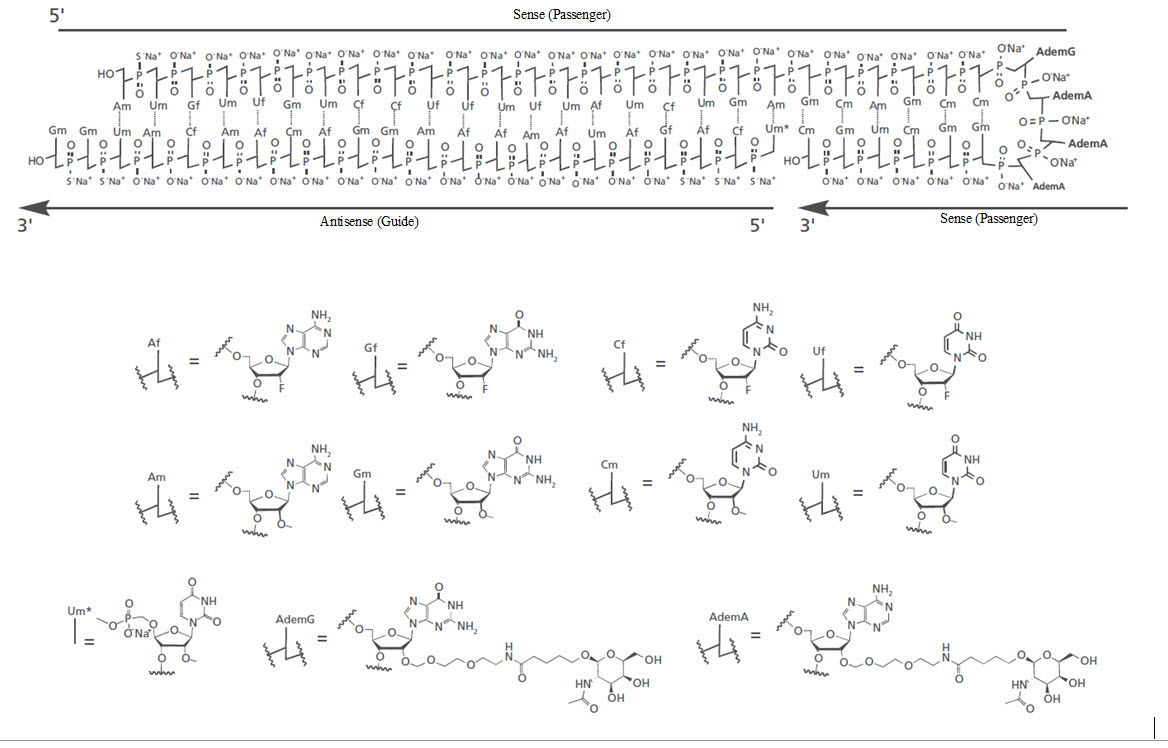
The molecular formula of nedosiran sodium is C
RIVFLOZA Pre-filled Syringe is supplied as a clear, sterile, preservative-free, colorless‑to‑yellow solution for subcutaneous injection containing either the equivalent of 160 mg (present as 170 mg nedosiran sodium salt) nedosiran in 1 mL or the equivalent of 128 mg (present as 136 mg nedosiran sodium salt) nedosiran in 0.8 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
RIVFLOZA vial is supplied as a clear, sterile, preservative-free, colorless-to-yellow solution for subcutaneous injection containing the equivalent of 80 mg (present as 85 mg nedosiran sodium salt) nedosiran in 0.5 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
5PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Instruct patients/caregivers on the appropriate dose of RIVFLOZA to use, the timing of the dose, how and where to inject subcutaneously, and what to do if a dose is missed.
For more information contact:
Novo Nordisk Inc.
Manufactured by
6PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 128 mg/0.8 mL
NDC: 0169-5307-08
List 530708
rivfloza
(nedosiran) injection
128 mg/0.8 mL
For subcutaneous injection only
1 x 0.8 mL Sterile Single-dose Pre-filled Syringe
Do not use the Pre-filled Syringe if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company

7PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 160 mg/mL
NDC: 0169-5306-10
List 530610
rivfloza
(nedosiran) injection
160 mg/mL
For subcutaneous injection only
1 x 1 mL Sterile Single-dose Pre-filled Syringe
Do not use the Pre-filled Syringe if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company

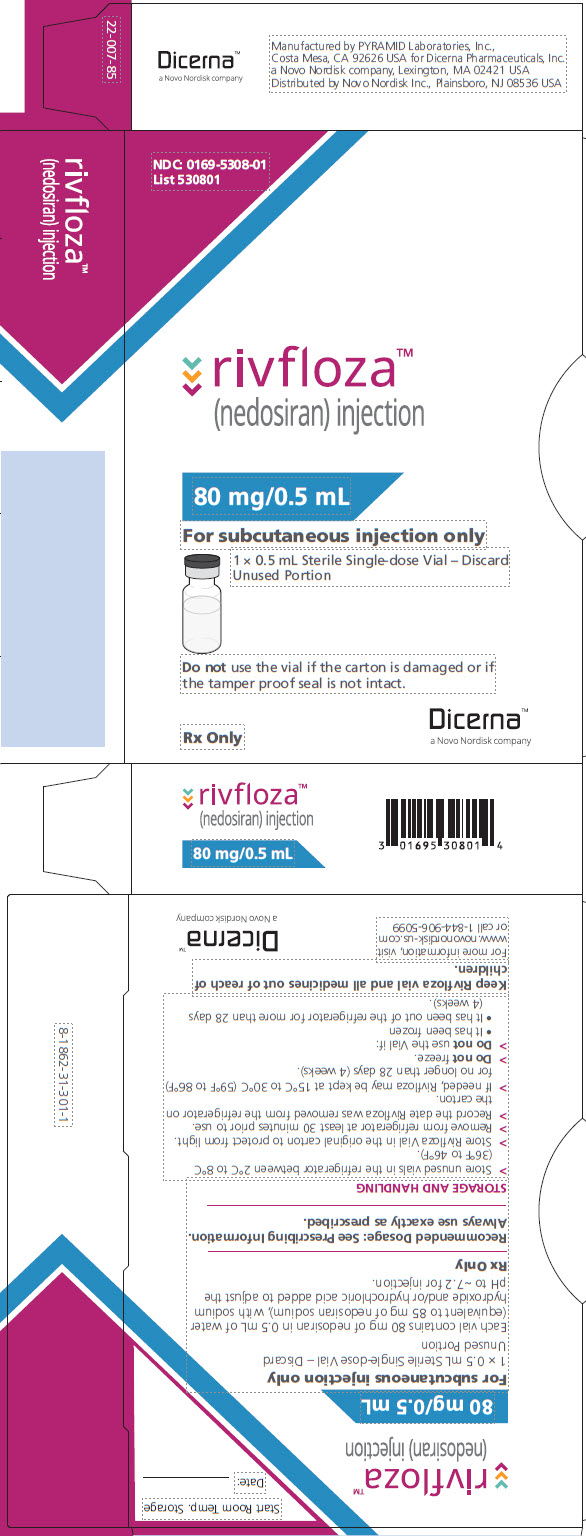
8PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Vial 80 mg/0.5 mL
NDC: 0169-5308-01
List 530801
rivfloza
(nedosiran) injection
80 mg/0.5 mL
For subcutaneous injection only
1 x 0.5 mL Sterile Single-dose Vial – Discard Unused Portion
Do not use the vial if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company