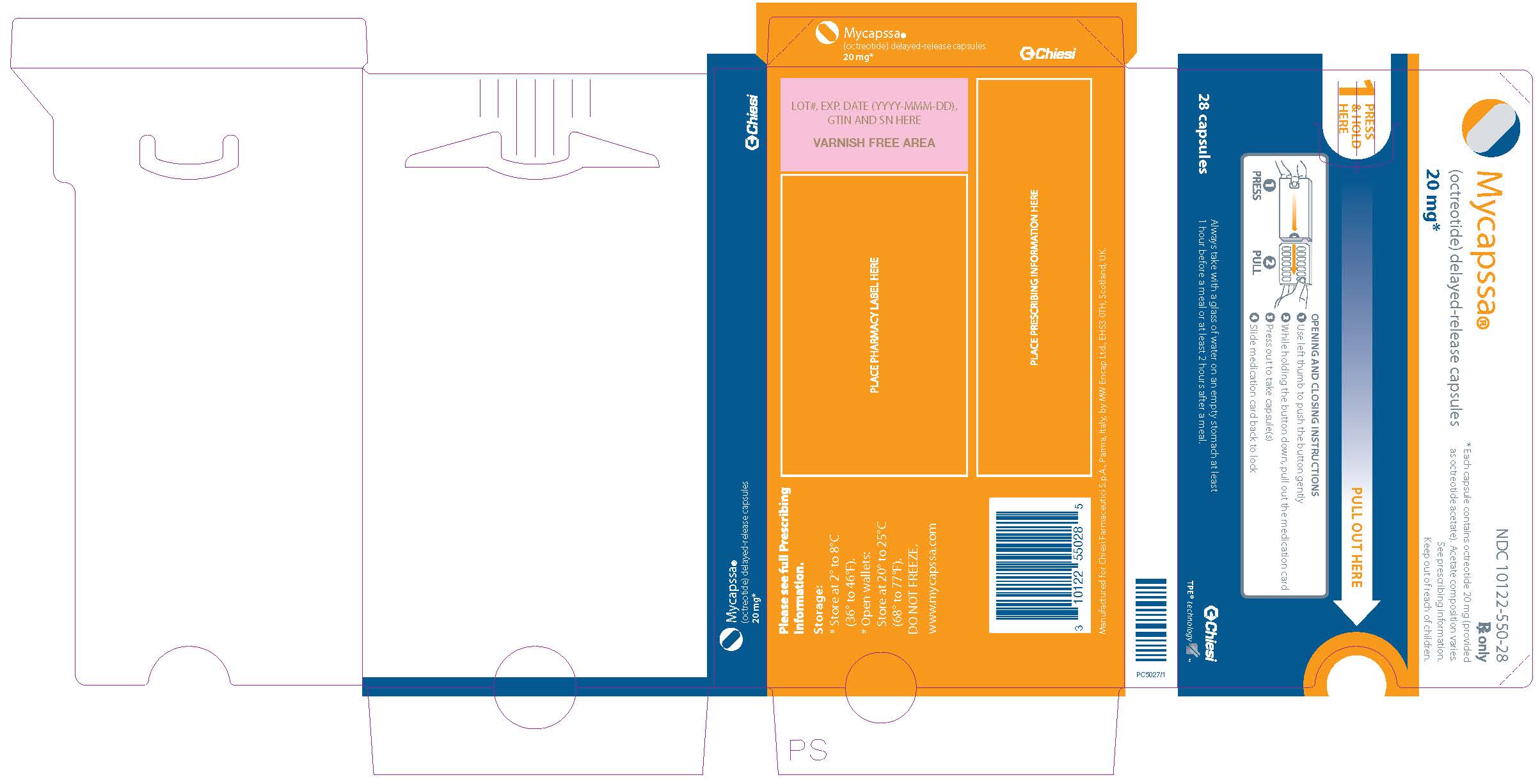
Mycapssa
What is Mycapssa (Octreotide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Between 10% and 15% of patients with endogenous hypercortisolism (Cushing syndrome) have ectopic (non-pituitary) production of adrenocorticotropin hormone (ACTH) that causes cortisol excess. In approximately 50% of these patients, the tumoral source of ACTH cannot be found initially despite very detailed and extensive imaging, including studies such as computed tomography, magnetic resonance imagi...
Summary: The goal of this study is to learn about how the hormone insulin controls blood sugar in a variety of people. The main question it aims to answer is about how much insulin the body actually needs to maintain a normal blood sugar level. Participants will be asked to come in for a one-day study visit in which they will undergo a graded insulin suppression test (GIST). The GIST involves intravenous (...
Summary: The PANENCA trial aims to reduce postoperative complications for patients who need a pancreatoduodenectomy (also known as a Whipple procedure), which is a complex operation to remove a tumor from or near the head of the pancreas. One of the most serious and common complications after this surgery is a leak from the pancreas, called a postoperative pancreatic fistula (POPF). Such leaks can cause in...
Related Latest Advances
Brand Information
- Cholelithiasis and Complications of Cholelithiasis
- Hyperglycemia and Hypoglycemia
- Thyroid Function Abnormalities
- Cardiac Function Abnormalities
- Steatorrhea and Malabsorption of Dieatary Fats
- Changes in Vitamin B
- Blood and lymphatic: pancytopenia, thrombocytopenia
- Cardiac: myocardial infarction, cardiac arrest, atrial fibrillation
- Ear and labyrinth: deafness
- Endocrine: diabetes insipidus, adrenal insufficiency in patients 18 months of age and under, pituitary apoplexy
- Eye: glaucoma, visual field defect, scotoma, retinal vein thrombosis
- Gastrointestinal: intestinal obstruction, peptic/gastric ulcer, abdomen enlarged
- General and administration site: generalized edema, facial edema
- Hepatobiliary: gallbladder polyp, fatty liver, hepatitis
- Immune: anaphylactoid reactions including anaphylactic shock
- Infections and infestations: appendicitis
- Laboratory abnormalities: increased liver enzymes, CK increased, creatinine increased
- Metabolism and nutrition: diabetes mellitus
- Musculoskeletal: arthritis, joint effusion, Raynaud's syndrome
- Nervous System: convulsions, aneurysm, intracranial hemorrhage, hemiparesis, paresis, suicide attempt, paranoia, migraines, Bell's palsy, aphasia
- Renal and urinary: renal failure, renal insufficiency
- Reproductive and breast: gynecomastia, galactorrhea, libido decrease, breast carcinoma
- Respiratory: status asthmaticus, pulmonary hypertension, pulmonary nodule, pneumothorax aggravated
- Skin and subcutaneous tissue: urticaria, cellulitis, petechiae
- Vascular: orthostatic hypotension, hematuria, gastrointestinal hemorrhage, arterial thrombosis of the arm
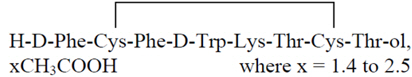
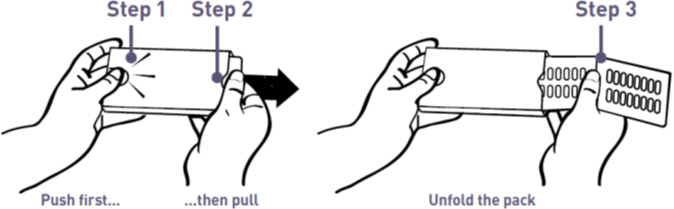
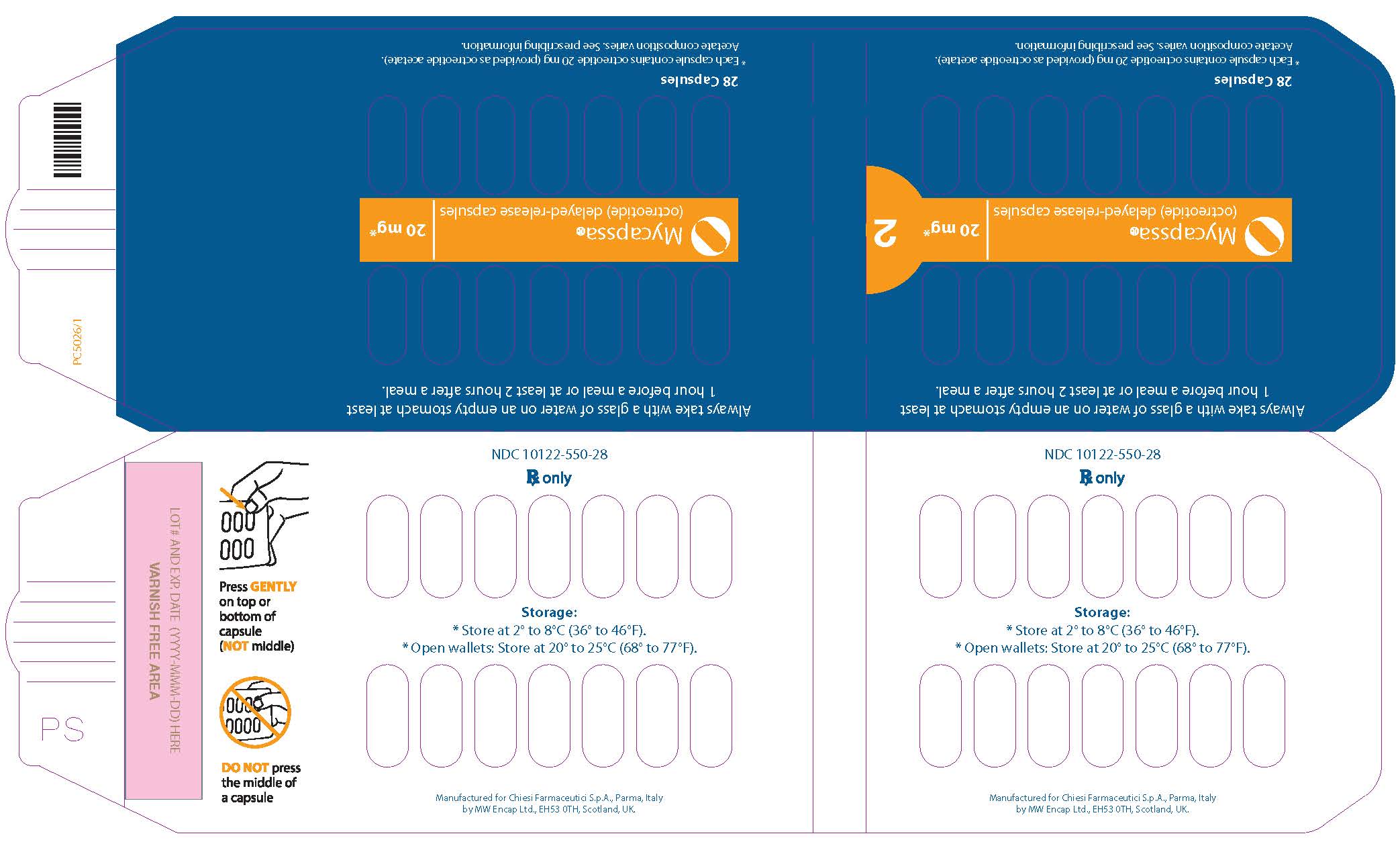
- Each MYCAPSSA wallet contains twenty-eight 20-mg capsules. The number of wallets required in a 28-day period depends on your prescribed dose.
- Each MYCAPSSA wallet has a locking mechanism that helps to keep the medicine away from children.
- Become familiar with using the MYCAPSSA wallet so you will know how to use it the right way.
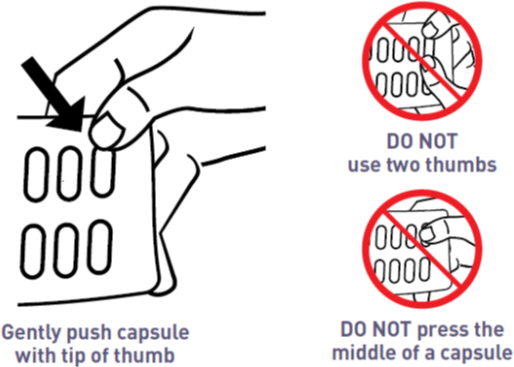
- Place the tip of a thumb at the edge of a capsule's plastic cavity (see
- Gently push the capsule until it is removed. Collect the removed capsule in your hand.
- Do not use two thumbs to push a capsule as this could damage it.
- Do not press the middle of a capsule. This could also damage it.
- If a capsule is cracked or broken, throw it away (discard it) and remove another capsule.
- Use left thumb to push the button gently
- While holding the button down, pull out the medication card
- Press out to take capsule(s)
- Slide medication card back to lock