Tivdak
What is Tivdak (Tisotumab Vedotin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: TIVDAK is used for the treatment of cervical cancer that has come back after chemotherapy. Chemotherapy is a treatment that uses medicines to stop the growth of cancer cells. This is done either by killing the cells or by stopping them from growing. The purpose of this study is to learn about possible side effects of TIVDAK, specially to any side effect that is related to the eye. A side effect is...
Summary: This study will have two phases: a sacituzumab tirumotecan safety run-in and a Phase 3 portion. The safety run-in phase will be used to evaluate the efficacy and safety of sacituzumab tirumotecan at the dose for evaluation in the Phase 3 portion. The purpose of this study is to compare the efficacy and safety of sacituzumab tirumotecan versus treatment of physician's choice as second-line treatmen...
Related Latest Advances
Brand Information
- TIVDAK can cause severe ocular toxicities resulting in changes in vision, including severe vision loss, and corneal ulceration.
- Conduct an ophthalmic exam, including an assessment of ocular symptoms, visual acuity, and slit lamp exam of the anterior segment of the eye prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated.
- Adhere to the required premedication and eye care before, during, and after infusion.
- Withhold TIVDAK until improvement and resume, reduce the dose, or permanently discontinue, based on severity
- Ocular Adverse Reactions
- Peripheral Neuropathy
- Hemorrhage
- Pneumonitis
- Severe Cutaneous Adverse Reactions

- “OSHA Hazardous Drugs.” OSHA.
- Inform patients about the eye exam they will receive with an eye care provider before starting TIVDAK, before each of the first nine cycles and as clinically indicated
- Inform patients about the ocular signs or symptoms the eye care provider will review before each cycle
- Inform patients that ocular adverse reactions may occur during treatment with TIVDAK and to contact their healthcare provider if they experience new or worsening ocular signs and symptoms
- Instruct patients to bring their eye drops to each infusion and advise on how to administer the eye drops throughout treatment
- Inform patients to avoid wearing contact lenses during treatment unless directed by an eye care provider
- Advise patients to report to their healthcare provider any numbness and tingling of the hands or feet or muscle weakness
- Instruct patients to contact their healthcare provider to seek immediate medical attention for signs or symptoms of unusual severe bleeding or hemorrhage
- Advise patients to immediately report new or worsening respiratory symptoms
- Inform patients of the signs and symptoms of severe cutaneous adverse reactions, including life-threatening and potentially fatal SJS, which include target lesions, worsening skin reactions, blistering or peeling of the skin, painful sores in mouth, nose, throat, or genital area, fever or flu-like symptoms, and swollen lymph nodes
- Instruct patients to contact their healthcare provider to seek immediate medical attention for signs or symptoms of severe cutaneous adverse reactions, including SJS
- Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise patients to inform their healthcare providers of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during treatment and for 2 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose
- Advise women not to breastfeed during treatment with TIVDAK and for 3 weeks after the last dose
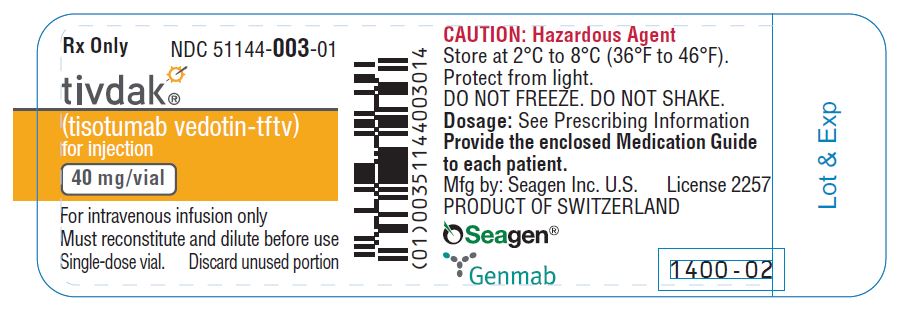
NDC 51144-003-01
tivdak®
(tisotumab vedotin-tftv)
for injection
40 mg/vial
CAUTION: Hazardous Agent
For intravenous infusion only
Must reconstitute and dilute before use
One single-dose vial.
Discard unused portion.
Provide the enclosed
Medication Guide to each patient
Seagen®
Genmab
Dosage:
See Prescribing Information.
Storage:
Store vial refrigerated at
2ºC to 8ºC (36ºF to 46ºF) in
original carton to protect from
light. DO NOT FREEZE.
DO NOT SHAKE.
No U.S. Standard of Potency
U.S. License 2257
TIVDAK and the TIVDAK logo
are US registered trademarks
owned by Seagen Inc.
 Seagen is US registered
Seagen is US registeredtrademark of Seagen Inc.
©2021 Seagen Inc. and Genmab.
All rights reserved.
Printed in USA
PRODUCT OF SWITZERLAND
Each vial contains 40 mg of
tisotumab vedotin-tftv.
No Preservative
After reconstitution with
4 mL of Sterile Water for
Injection, USP, the
concentration of TIVDAK
(tisotumab vedotin-tftv) is
10 mg/mL
Each mL of reconstituted
solution contains 10 mg of
tisotumab vedotin-tftv,
D-mannitol (30 mg),
L-histidine (2.11 mg),
L-histidine monojhydrochloride
(3.44 mg), and sucrose (30mg)
Mfg by:
Seagen Inc.
Bothell, WA 98021
For more information:
1-855-4SEAGEN
GTIN 00351144003014
1401 - 02
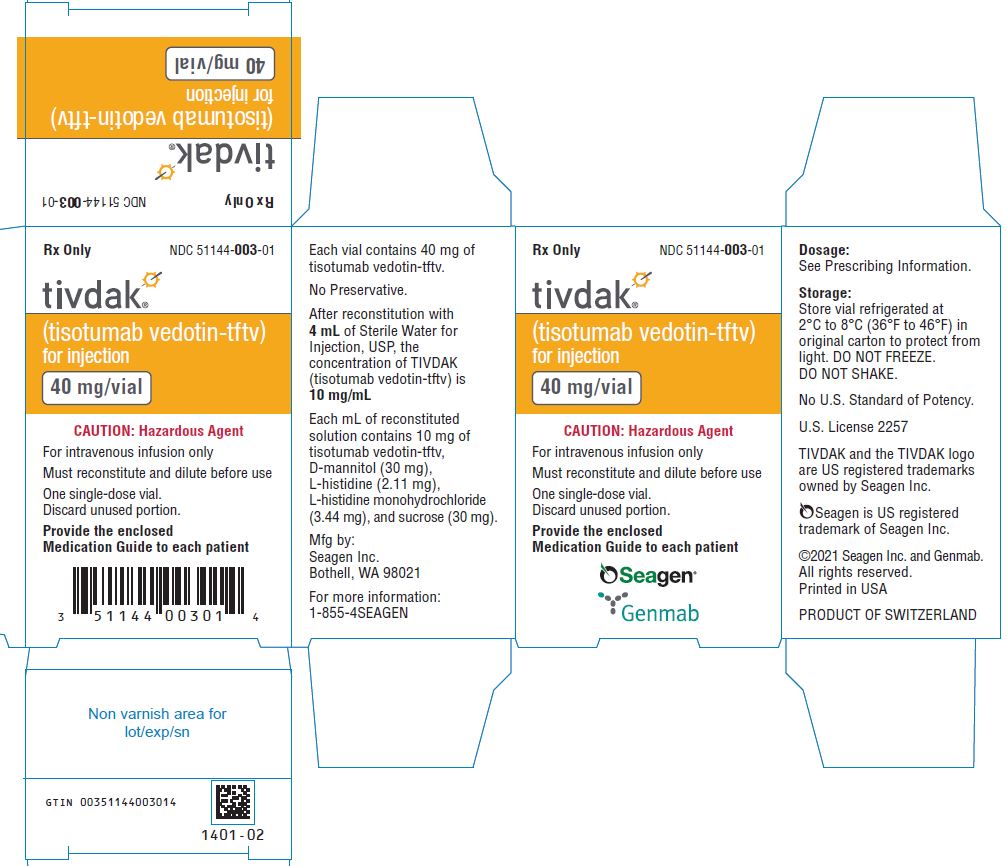
NDC 51144-003-01
tivdak®
(tisotumab vedotin-tftv)
for injection
40 mg/vial
For intravenous infusion only
Must reconstitute and dilute before use
Single-dose vial. Discard unused portion.
CAUTION: Hazardous Agent
Store at 2ºC to 8ºC (36ºF to 46ºF)
Protect from light.
DO NOT FREEZE. DO NOT SHAKE.
Dosage: See Prescribing Information
Provide the enclosed Medication Guide to each patient.
Mfg by: Seagen Inc. U.S. License 2257
PRODUCT OF SWITZERLAND
Seagen®
Genmab
1400-02