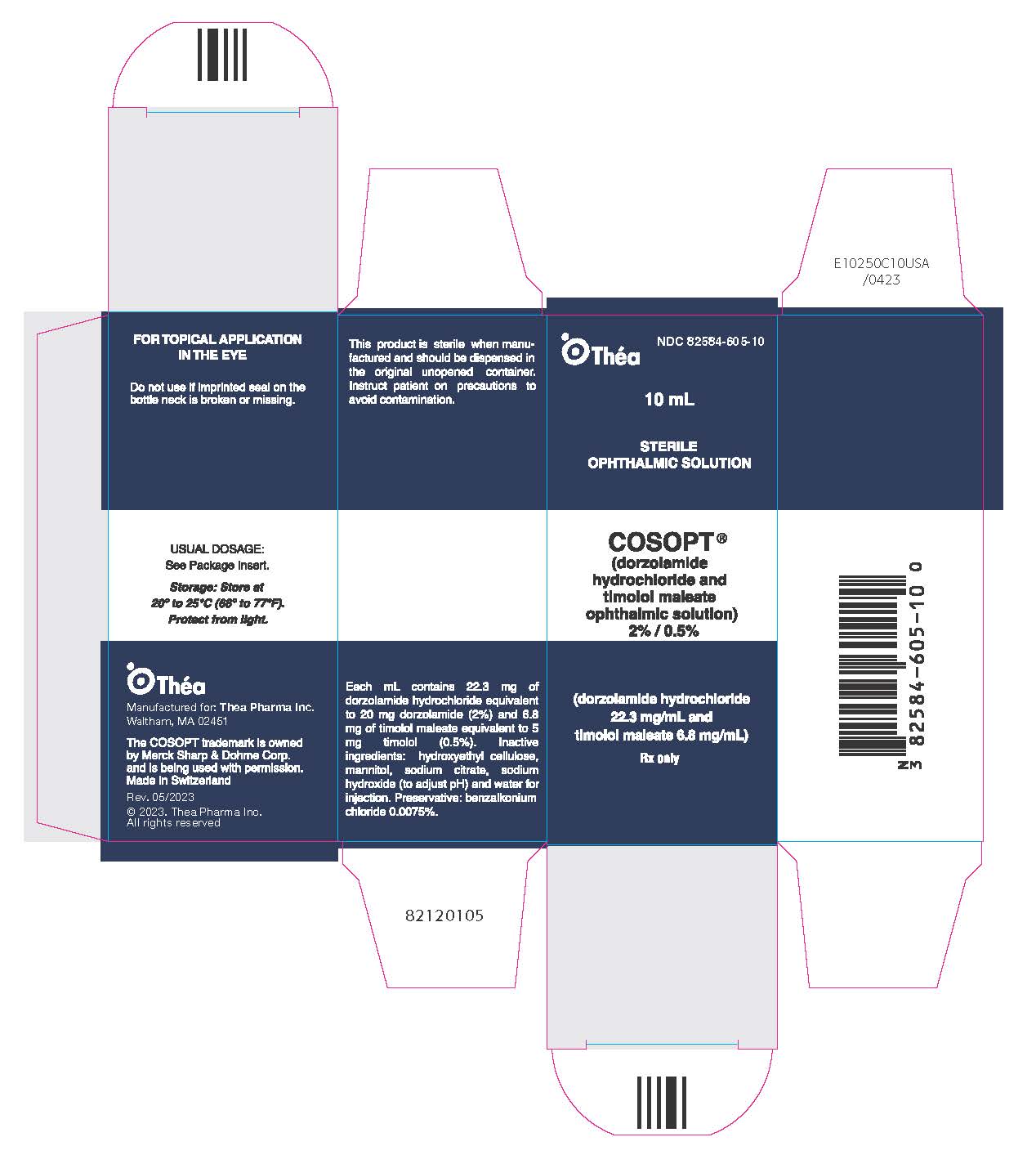
Cosopt
What is Cosopt (Dorzolamide)?
For many people living with glaucoma or ocular hypertension, preserving vision is not just a medical goal, it’s a matter of maintaining independence, confidence, and quality of life. Cosopt (dorzolamide and timolol) is an eye drop combination therapy designed to lower high pressure inside the eye, a leading cause of optic nerve damage and vision loss. By reducing intraocular pressure (IOP), this medication helps protect eyesight and slow the progression of glaucoma, offering reassurance and long-term visual stability for patients.
Cosopt belongs to a class of drugs known as carbonic anhydrase inhibitors (from dorzolamide) combined with a beta-blocker (timolol). This dual-action formula has been widely used for decades as a second-line or adjunct treatment for glaucoma when single medications do not adequately control eye pressure. Its well-established safety profile and proven effectiveness make it a trusted option in ophthalmic care.
What does Cosopt do?
Cosopt (dorzolamide and timolol) is primarily prescribed to treat elevated intraocular pressure in individuals with open-angle glaucoma or ocular hypertension. These conditions occur when the natural fluid (aqueous humor) inside the eye builds up, causing pressure that can damage the optic nerve, the nerve responsible for vision.
By lowering this pressure, Cosopt helps prevent gradual vision loss and blindness. While it cannot restore vision already lost to glaucoma, maintaining healthy eye pressure significantly slows disease progression. Many patients report improved comfort, reduced eye strain, and peace of mind knowing that their treatment is effectively managing a chronic condition.
Clinical studies have shown that the combination of dorzolamide and timolol lowers eye pressure more effectively than either medication alone, making it a powerful choice for patients requiring stronger or combination therapy (FDA, 2023).
How does Cosopt work?
Cosopt works through two complementary mechanisms that target the main cause of high eye pressure, excess fluid inside the eye.
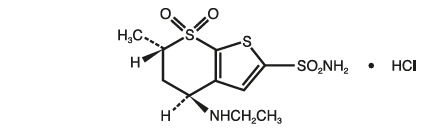
- Dorzolamide, the first component, is a carbonic anhydrase inhibitor. It reduces the production of aqueous humor (the clear fluid inside the eye) by blocking an enzyme responsible for fluid formation.
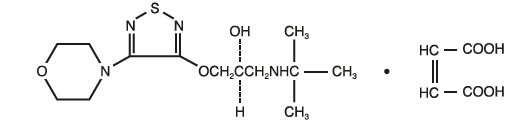
- Timolol, the second component, is a beta-blocker that also decreases fluid production but does so by slowing the action of certain nerve signals in the eye.
These actions decrease eye fluid, lowering intraocular pressure (IOP). Lowering IOP is crucial as prolonged high pressure damages the optic nerve, causing permanent vision loss. Cosopt’s dual-action effectively lowers IOP for patients needing more than one medication, simplifying treatment by combining two drugs into one bottle, improving convenience and adherence.
Cosopt side effects
As with most eye medications, Cosopt can cause some side effects, though not everyone experiences them. Most are mild and temporary, occurring shortly after the drops are applied.
Common side effects include:
- Temporary burning, stinging, or discomfort in the eyes
- Blurred vision or watery eyes
- Unusual taste in the mouth after application
- Feeling of something in the eye
- Mild eye redness
These typically improve as your eyes adjust to the medication. To minimize unpleasant taste, gently close your eyes after applying the drops and press the inner corner of your eyelid for one minute, this limits the medication’s absorption into your system.
Serious but rare side effects may include:
- Shortness of breath, wheezing, or slow heartbeat (from timolol’s beta-blocker effects)
- Severe eye irritation, swelling, or pain
- Allergic reactions such as rash, itching, or swelling of the face or throat
Seek immediate medical care if you experience any breathing difficulties, chest pain, or vision changes.
Who should avoid Cosopt:
Avoid this medication if you have severe asthma, COPD, certain heart rhythm disorders, severe heart failure, or sulfa allergies. Inform your doctor about all medical conditions and medications.
Cosopt dosage
Cosopt, a sterile ophthalmic solution, is applied to the affected eye(s) once or twice daily as directed by a doctor. Each drop delivers dorzolamide and timolol directly to eye tissues, minimizing systemic absorption and side effects compared to oral medications.
To ensure safety and effectiveness, wash hands before use, avoid touching the dropper tip, and replace the cap tightly after each use.
Your eye doctor will regularly monitor intraocular pressure to confirm medication effectiveness. Periodic eye examinations, heart rate checks, and breathing assessments may also be recommended, especially for patients with heart or lung disease. Dosage adjustments or additional monitoring may be needed for older adults or those on multiple glaucoma medications.
Does Cosopt have a generic version?
Yes. A generic version of Cosopt (dorzolamide and timolol ophthalmic solution) is available and approved by the U.S. Food and Drug Administration (FDA). The generic contains the same active ingredients and works the same way as the brand-name drug.
FDA-approved generics match brand-name drugs in quality, strength, and safety, offering cost savings with equivalent clinical benefits. Cosopt PF (preservative-free) is available for those sensitive to preservatives, providing the same therapeutic effect in a gentler formulation.
Conclusion
Cosopt (dorzolamide and timolol) plays a crucial role in protecting vision for people with glaucoma or ocular hypertension. By combining two effective ingredients into one convenient eye drop, it helps lower eye pressure and reduces the risk of optic nerve damage, a key step in preserving long-term sight.
While side effects can occur, they are often mild and manageable with proper use and regular follow-up. Every patient’s response to treatment is unique, so consistent communication with your ophthalmologist is essential to optimize therapy and monitor eye health.
When taken as directed and monitored by a qualified healthcare provider, Cosopt offers a safe, proven, and effective way to maintain visual stability and safeguard one of life’s most valuable senses, your sight.
References
- Mayo Clinic. (2024). Dorzolamide and timolol (ophthalmic route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Dorzolamide and timolol ophthalmic: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- U.S. Food and Drug Administration (FDA). (2023). Approved Drug Products: Cosopt (dorzolamide and timolol). Retrieved from https://www.accessdata.fda.gov
- National Eye Institute (NIH). (2024). Facts about glaucoma and eye pressure management. Retrieved from https://www.nei.nih.gov
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- have or have had asthma.
- have chronic obstructive pulmonary disease (COPD) which emphysema, chronic bronchitis or both.
- have heart problems including a slow heartbeat, heart block, heart failure, or your heart muscle suddenly becomes weak due to a severe heart attack or other heart problem that caused heart damage (cardiogenic shock).
- are allergic to any of the ingredients in COSOPT. See the end of this Patient Information leaflet for a complete list of ingredients in COSOPT.
- have or have had allergies to sulfa drugs
- have a history of anaphylactic reactions to allergens
- have atopy (genetic disposition to develop allergic reactions)
- have or have had muscle weakness or myasthenia gravis
- have diabetes
- have thyroid disease
- have or have had kidney or liver problems
- plan to have any type of surgery
- wear contact lenses
- are using any other eye drops
- have an eye infection or eye trauma
- are pregnant or plan to become pregnant. It is not know if COSOPT will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while using COSOPT. You and your healthcare provider will decide if you should use COSOPT while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if COSOPT passes into breastmilk. Talk to your healthcare provider about the best way to feed your baby while using COSOPT.
- See the complete
- Use COSOPT exactly as your healthcare provider tells you.
- Use 1 drop of COSOPT in the affected eye or both eyes if needed, 2 times each day. 1 drop in the morning and 1 drop in the evening.
- If you are using COSOPT with another eyedrop,
- If you have eye surgery or have any problems with your eye such as trauma or an infection, talk to your healthcare provider about continuing to use the bottle (multidose) that contains COSOPT.
- COSOPT contains a preservative called benzalkonium chloride. The preservative may be absorbed by soft contact lenses. If you wear contact lenses, remove them before using COSOPT. The lenses can be placed back into your eyes 15 minutes after using COSOPT.
- Do not touch your eye or eyelid with the dropper tip. Eye medicines, not handled the right way, can become contaminated by bacteria that can cause eye infections. Serious damage to the eye and followed by loss of vision may happen from using contaminated eye medicines. If you think your COSOPT medicine may be contaminated, or if you develop an eye infection, contact your healthcare provider right away about continuing to use your bottle of COSOPT.
- If you use too much COSOPT you may have dizziness, headaches, shortness of breath, slow heartbeats, or problems breathing. If you have any of these symptoms call your healthcare provider or go to the nearest hospital emergency room right away.
- severe breathing problems. These breathing problems can happen in people who have asthma, chronic obstructive pulmonary disease, or heart failure and can cause death. Tell your healthcare provider right away if you have breathing problems while using COSOPT.
- heart failure. This can happen in people who already have heart failure and in people who have never had heart failure before. Tell your healthcare provider right away if you get any of these symptoms of heart failure while taking COSOPT:
- shortness of breath
- irregular heartbeat (palpitations)
- swelling of your ankles or feet
- sudden weight gain
- serious sulfa (sulfonamide) reactions. Serious reactions including death can happen in people who are allergic to sulfonamide medicines like one of the medicines in COSOPT. Other serious reactions can include:
- severe skin reactions
- liver problems
- blood problems
- swelling of your face, lips, mouth, or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest
- sweating
- increased allergic reactions. People who have a genetic history of developing allergies (atopy) or who have a history of severe anaphylactic reactions from different allergens may have increased allergic reactions while taking beta-blockers, like one of the medicines in COSOPT. Your usual dose of epinephrine used to treat your anaphylactic reactions may not work as well. Stop using COSOPT and call your healthcare provider or get emergency help right away if you get any of these symptoms of an allergic reaction:
- swelling of your face, lips, mouth or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest
- sweating
- worsening muscle weakness. Muscle weakness symptoms including double vision or drooping eyelids can happen while using COSOPT. Muscle weakness can get worse in people who already have problems with muscle weakness like myasthenia gravis.
- swelling of eye. Some people with low counts of certain types of cells in the eye have developed corneal edema when using COSOPT. Call your healthcare provider if you have swelling in your eyes.
- eye burning
- eye stinging
- eye redness
- blurred vision
- eye tearing
- eye itching
- a bitter, sour, or unusual taste after putting in your eyedrops
- Store at 68° to 77°F (20° to 25°C).
- Protect from light.
- Do not use COSOPT after the expiration date on the bottle.
- COSOPT is for use in the eye.
- If you are using COSOPT with another eyedrop,
- If you wear contact lenses, remove them before using COSOPT. The lenses can be placed back into your eyes
- Do not touch your eye or eyelid with the dropper tip. Eye medicines, not handled the right way, can become contaminated by bacteria that can cause eye infections. Serious damage to the eye and followed by loss of vision may happen from using contaminated eye medicines. If you think your COSOPT medicine may be contaminated, or if you develop an eye infection, contact your healthcare provider right away about continuing to use your bottle of COSOPT.
- Wash your hands before each use to make sure you do not infect your eyes while using COSOPT.
- Before using the eyedrops for the first time, be sure the Safety Seal around the cap is not broken. If the Safety Seal is broken, call your pharmacist to get a new bottle of COSOPT.
- The dropper tip is made to give a single drop of COSOPT.
- After you have used all of your doses of COSOPT, there will be some COSOPT left in the bottle.
- There is an extra amount of COSOPT that has been added to the bottle. You will get the full amount of COSOPT that your doctor prescribed.
- Do not try to remove the extra COSOPT medicine from the bottle.
Rev. 05/2023