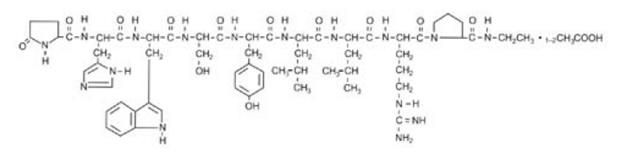
Leuprolide Acetate
What is Lupron (Leuprolide Acetate)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this open-label, multicenter, phase IIIb, single-arm study is to characterize the efficacy and safety of the combination of ribociclib and standard adjuvant endocrine therapy (ET) on invasive breast cancer-free survival (iBCFS), in a close to clinical practice patient population with HR-positive (HR+), HER2-negative (HER2-), Anatomic Stage Group III, IIB, and a subset of Stage IIA E...
Summary: This is a prospective, open-label, multi-center seamless phase II to phase III randomized clinical trial designed to compare SST with or without PET-directed local therapy in improving the castration-resistant prostate cancer-free survival (CRPC-free survival) for Veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1-10 sites of metastatic disease based on the clinica...
Summary: This research study is studying a combination of HER2-directed therapies (trastuzumab and pertuzumab) and hormonal therapy as a treatment after surgery for hormone receptor positive breast cancer. The study drugs involved in this study are: * A combination of trastuzumab and pertuzumab given as an injection under the skin (PHESGO) * Hormonal (endocrine) Treatment
Related Latest Advances
Brand Information
- Hypersensitivity to gonadotropin-releasing hormone (GnRH), GnRH agonist analogs, including leuprolide acetate, or any of the excipients in LUPRON DEPOT 3.75 mg
- Undiagnosed abnormal uterine bleeding
- Pregnancy
- Loss of Bone Mineral Density
- Severe Cutaneous Adverse Reactions
- Hypersensitivity Reactions
- Initial Flare of Symptoms with Management of Endometriosis
- Convulsions
- Clinical Depression
- Skin and Subcutaneous Tissue: rash, urticaria, photosensitivity, erythema multiforme, bullous dermatitis, dermatitis exfoliative, drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), and acute generalized exanthematous pustulosis (AGEP)
- Body as a whole: Hypersensitivity reactions including anaphylaxis
- Nervous/Psychiatric System: Mood swings, including depression; suicidal ideation and attempt; convulsion, peripheral neuropathy, paralysis
- Hepato-biliary system: Serious liver injury
- General disorders and administration site conditions: Injection site reactions including induration, abscess, and necrosis
- Injury, poisoning and procedural complications: Spinal fracture
- Investigations: Decreased white blood count
- Musculoskeletal and connective tissue system: Tenosynovitis-like symptoms
- Vascular system: Hypotension, hypertension, deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke, transient ischemic attack
- Respiratory system: Symptoms consistent with an asthmatic process
- Multi-system disorders: Symptoms consistent with fibromyalgia (e.g., joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath), individually and collectively.
- one prefilled dual-chamber syringe
- one plunger
- two alcohol swabs
- Advise females of reproductive potential of the possible risk to a fetus. Advise patients to inform healthcare provider of a known or suspected pregnancy
- If contraception is indicated, advise females of reproductive potential to use non-hormonal contraception during treatment with LUPRON DEPOT 3.75 mg
