Brand Name
Revcovi
Generic Name
Elapegademase-Lvlr
View Brand Information FDA approval date: October 05, 2018
Form: Injection
What is Revcovi (Elapegademase-Lvlr)?
REVCOVI is indicated for the treatment of adenosine deaminase severe combined immune deficiency in pediatric and adult patients. REVCOVI is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency in pediatric and adult patients.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Revcovi (elapegademase-lvlr)
1INDICATIONS AND USAGE
REVCOVI is indicated for the treatment of adenosine deaminase severe combined immune deficiency (ADA-SCID) in pediatric and adult patients.
2DOSAGE FORMS AND STRENGTHS
Injection: 2.4 mg/1.5 mL (1.6 mg/mL) clear and colorless solution of elapegademase-lvlr in a single-dose vial.
3CONTRAINDICATIONS
None.
4DRUG INTERACTIONS
The drug interaction potential of REVCOVI is not known.
5OVERDOSAGE
There are no reports of administration of REVCOVI in excess of the prescribed doses. The highest weekly prescribed dose administered in the clinical studies was 0.4 mg/kg. In nonclinical studies, there was no evidence of toxicity related to study drug at doses up to 1.8-fold the clinical dose (based on mean human AUC normalized to the dose of REVCOVI administered per patient), except for a slight increase in activated partial thromboplastin time (APTT).
6DESCRIPTION
Elapegademase-lvlr is a recombinant adenosine deaminase (rADA) based on bovine amino acid sequence, conjugated to monomethoxypolyethylene glycol (mPEG). rADA is manufactured in
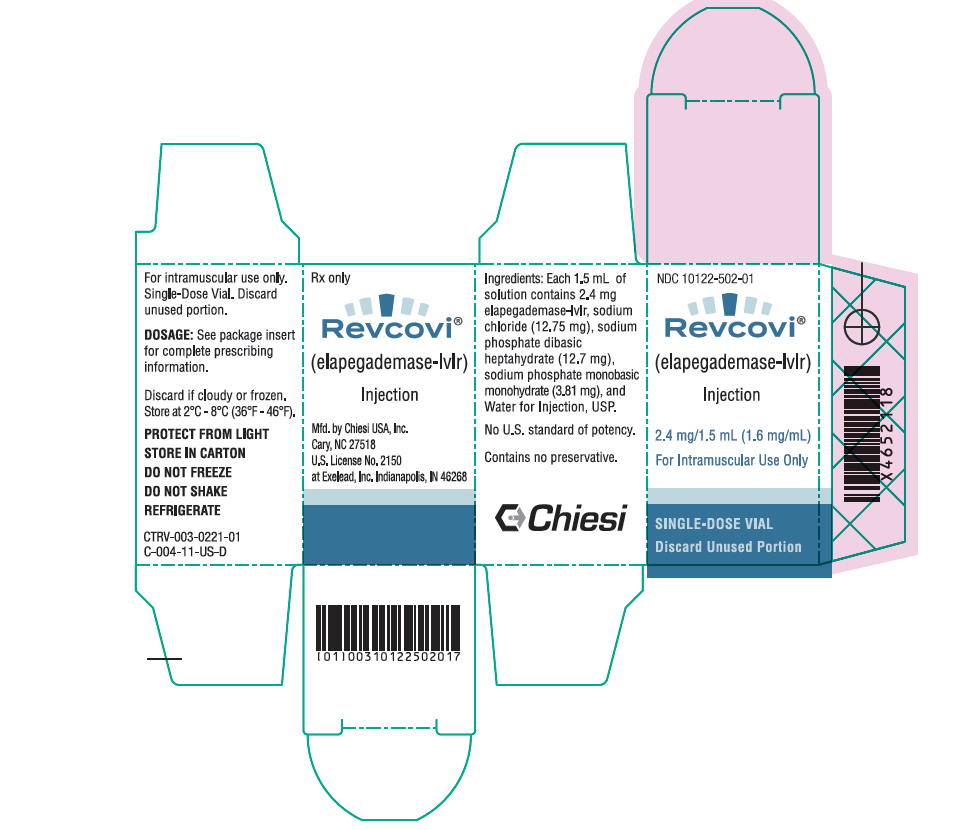
REVCOVI (elapegademase-lvlr) injection is a sterile, preservative free, clear, colorless solution for intramuscular use supplied in single-dose vials. Each vial provides 1.5 mL of solution containing 2.4 mg elapegademase-lvlr (1.6 mg/mL), sodium chloride (12.75 mg), sodium phosphate dibasic heptahydrate (12.7 mg), sodium phosphate monobasic monohydrate (3.81 mg), and Water for Injection, USP. The pH is 6.9.
7HOW SUPPLIED/STORAGE AND HANDLING
REVCOVI (elapegademase-lvlr) injection, 2.4 mg/1.5 mL (1.6 mg/mL), is a sterile, preservative free, clear, colorless solution for intramuscular use available as one single-dose vial per carton (NDC 10122-502-01).
The vial stopper is not made with natural rubber latex.
Single-dose vial; do not re-use the vial. Discard unused portions.
Store REVCOVI in the refrigerator between 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or shake. REVCOVI should not be used if there are any indications that it may have been frozen.
8PATIENT COUNSELING INFORMATION
Importance of Compliance
Counsel patients and caregivers that continuous therapy and adherence to the recommended drug schedule is important for the success of the treatment.
Manufactured by: Chiesi USA, Inc. Cary, NC 27518, USA, U.S. License No. 2150 at Exelead Inc., 6925 Guion Rd, Indianapolis, IN 46268, USA
CTRV-001-1120-00-SPL-2
9PRINCIPAL DISPLAY PANEL - 2.4 mg/1.5 mL Vial Carton
NDC10122-502-01
Revcovi
2.4 mg/1.5 mL (1.6 mg/mL)
For Intramuscular Use Only
SINGLE-DOSE VIAL
Discard Unused Portion